RetinAI Receives FDA Clearance for Discovery Image Management Platform for Ophthalmology

RetinAI Medical AG announced it has received FDA 510(k) clearance for RetinAI Discovery, the company’s image and data management platform for interoperability across the clinical and clinical study ecosystems in ophthalmology.
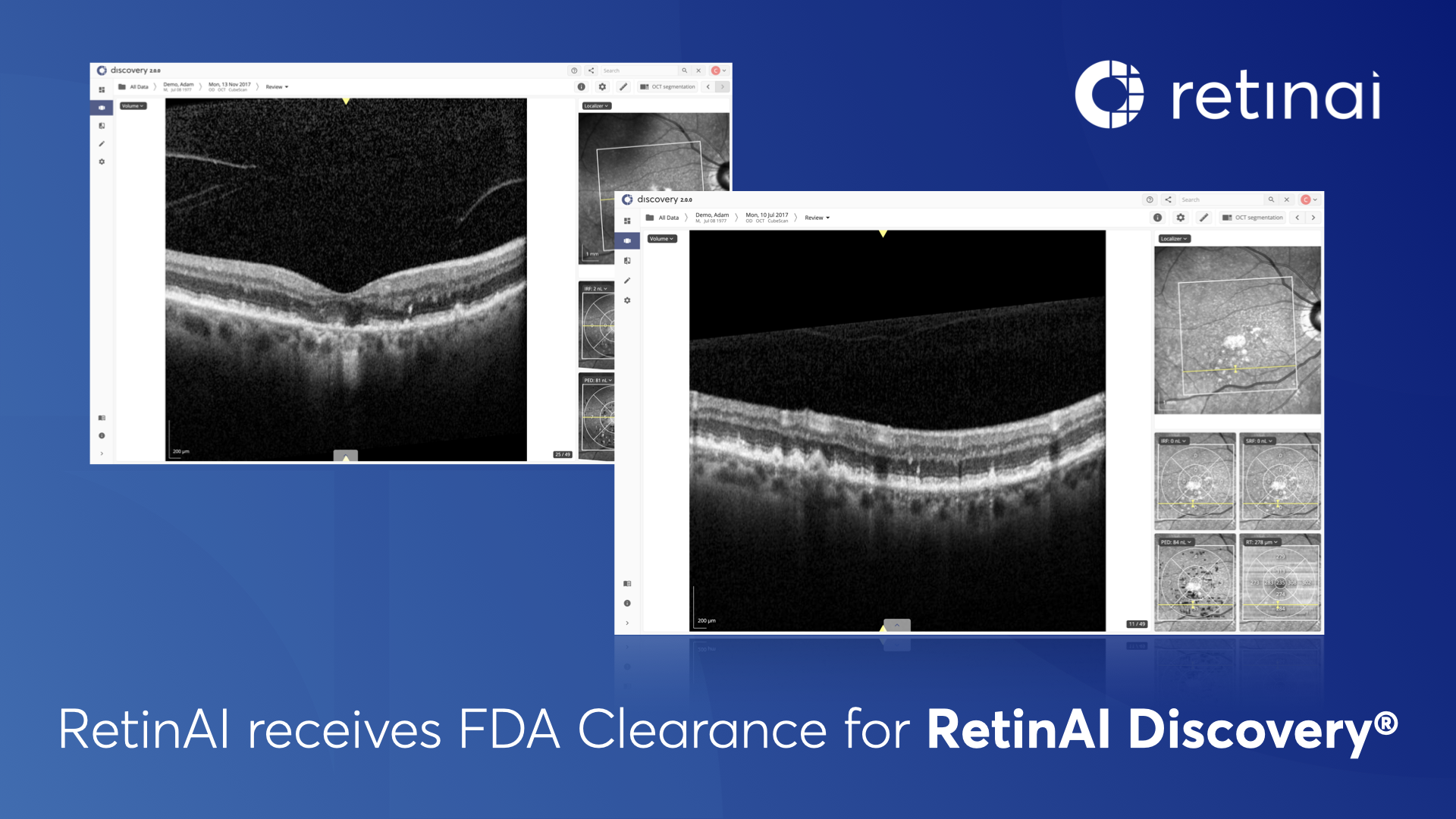
The Discovery platform can be used to organize and analyze ophthalmic medical images, such as optical coherence tomography (OCT) scans and fundus photographs, from a variety of medical devices. The platform provides secure, on the cloud, web-based access to facilitate collaborative and interactive analysis of clinical cases in real-time.
Features such as multi-modal imaging compatibility, comparison views of clinical visits, deployment of grading surveys, and electronic case report forms (eCRFs) for collecting clinical data are all integrated into the platform. These features were developed to improve workflows and enable the right decisions sooner during treatment and analyses in clinical practice and clinical studies.
In addition to the Discovery platform, RetinAI has developed a set of AI models to quantify fluid and layer segments in retinal tissue with expert-level accuracy in vision-threatening conditions such as age-related macular degeneration (AMD), diabetic retinopathy (DR), diabetic macular edema (DME) and retinal vein occlusion (RVO). These AI models are CE-marked, and the company is planning an FDA submission in 2022. Once approved, these AI models will be integrated into Discovery for automated analysis of key disease markers to aid physicians in their clinical decisions.
“Discovery is an advancement in image management platforms for ophthalmology and is designed to deliver transparency across datasets from different devices," RetinAI’s CEO Carlos Ciller, PhD, said in a company news release. "With its intuitive user interface, and web-based access, Discovery fosters participative medical management to better serve patients and speed up decision making in clinical studies. We’ve already seen the benefits of how Discovery streamlines management and analysis in clinical studies and clinics in Europe, and we are excited to bring our innovation to the U.S.”
