GenSight Biologics Announces Initial Results from New Meta-Analyses on Visual Outcomes with Lumevoq Gene Therapy at NANOS 2024

GenSight Biologics announced initial results of new meta-analyses in Leber Hereditary Optic Neuropathy (LHON), which showed those treated with Lumevoq (GS010; lenadogene nolparvovec) gene therapy experienced a rate of visual recovery greater than that of idebenone-treated patients and untreated (natural history) patients. The meta-analyses are the first to focus solely on patients with the m.11778G>A ND4 mutation, which is the most common mutation and one with a poor visual prognosis.[1]
Figure 1. Visual Recovery (CRR1 from Nadir2) Among ND4 LHON Patients – Results from Three New Meta-Analyses [3,4]
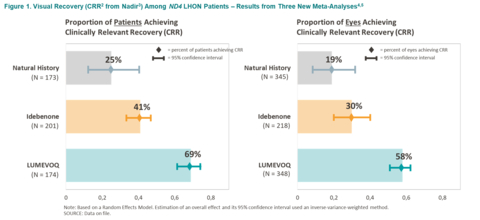
The meta-analyses depict a gradient of efficacy of visual recovery with Lumevoq intravitreal gene therapy resulting in greater recovery rates than that of idebenone treatment, and both greater than that in the natural history of the disease. This gradient of recovery, based on the CRR measure of visual improvement, is observed at both eye level and patient level (response in one or both eyes). There is no overlap in confidence intervals when Lumevoq is compared to idebenone and to natural history, indicating a positive difference in visual outcomes.
The results were presented at the 2024 annual meeting of the North American Neuro-Ophthalmology Society (NANOS) and will also be presented at other major ophthalmology, neurology and neuro-ophthalmology medical conferences in Europe and the US later this year.
Nancy J. Newman, MD, LeoDelle Jolley Professor of Ophthalmology and Neurology at the Emory University School of Medicine in Atlanta, United-States, presented the findings at NANOS 2024.
“This study is very important in order to compare these therapies’ abilities to achieve equivalent end-point metrics. It is clear that the degree of efficacy of the gene therapy was greater than the other currently available options of no treatment and idebenone use as previously administered in studies," Dr. Newman said in a company news release.
Based on the pre-defined statistical analysis plan, the three meta-analyses were performed on three groups of ND4-LHON patients according to therapy: Lumevoq gene therapy one-time intravitreal injection, idebenone therapy, and no treatment (natural history). To be included in the meta-analyses, studies had to satisfy a number of criteria that ensured comparability: for example, at least 3 data points per eye, information on the type of mutation with inclusion of ND4 patients only, and availability of visual outcomes data such as CRR. Clinically Relevant Recovery (CRR) is an accepted criterion of clinically meaningful visual improvement in LHON patients6 and could be extracted from all three patient samples. The final set of qualifying studies consisted of five natural history studies (173 patients), six idebenone studies (201 patients) and three LUMEVOQ® studies (174 patients).
The company is currently engaging with authorities in the US, EU, and UK to align on the regulatory path for Lumevoq. The company aims to restart the Early Access Program (AAC) in France in Q3 2024. Follow-up of patients in the phase 3 REFLECT study of Lumevoq is ongoing, with topline results at year 4 of follow-up expected this month.
About GenSight Biologics
GenSight Biologics S.A. is a clinical-stage biopharma company focused on discovering and developing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders. GenSight Biologics’ pipeline leverages two core technology platforms, the Mitochondrial Targeting Sequence (MTS) and optogenetics, to help preserve or restore vision in patients suffering from blinding retinal diseases. GenSight Biologics’ lead product candidate, GS010, is in Phase III trials in Leber Hereditary Optic Neuropathy (LHON), a rare mitochondrial disease that leads to irreversible blindness in teens and young adults. Using its gene therapy-based approach, GenSight Biologics’ product candidates are designed to be administered in a single treatment to each eye by intravitreal injection to offer patients a sustainable functional visual recovery.
References
1 Newman et al: J Neuro-Ophthalmol 2020 Dec;40(4):547-557.
2 “Clinically Relevant Recovery”, or CRR, denotes an improvement in Best-Corrected Visual Acuity (BCVA) that satisfies one of two conditions: (1) A 10-letter (≥0.2 LogMAR) improvement for an on-chart starting visual acuity. (2) Improvement from “off-chart” to “on-chart” (≤1.6 LogMAR).
3 Nadir = lowest observation of visual acuity between baseline and time point of interest.
4 Three meta-analyses pre-definedin a statistical analysis plan
5 Sources of data: For idebenone and natural history data, systematic review of the literature and available clinical/regulatory reports on ND4 LHON patients; for Lumevoq, all phase 3 studies (RESCUE/RESTORE, REVERSE/RESTORE and REFLECT).
6 Carelli et al: J Neuro-Ophthalmol 2017; 0: 1-11
