Atsena Therapeutics Unveils XLRS Gene Therapy Program Leveraging Novel Spreading Capsids
Atsena Therapeutics unveiled its preclinical gene therapy program for X-linked retinoschisis (XLRS), a monogenic disease caused by mutations in the RS1 gene. XLRS is characterized by schisis, or abnormal splitting of the layers of the retina, which causes impaired visual acuity that is not correctable and leads to progressive vision loss. XLRS primarily affects males and is typically diagnosed in early childhood.
“We are pleased to disclose our program for XLRS, an inherited retinal disease lacking approved treatments to improve or restore vision,” said Patrick Ritschel, MBA, Chief Executive Officer of Atsena. “Following a recent pre-IND meeting with the U.S. Food and Drug Administration, we are working expeditiously to advance our differentiated gene therapy candidate for XLRS toward the clinic.”
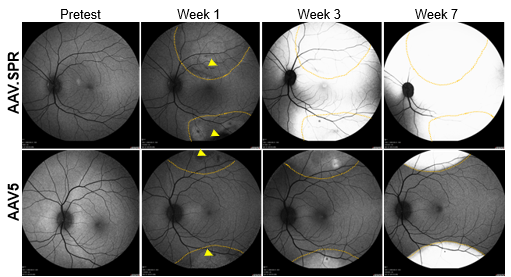
Atsena’s XLRS gene therapy program leverages one of the company’s novel AAV capsids, AAV.SPR, that spreads laterally beyond the subretinal injection site, enabling safe and efficient transduction of the central retina (where schisis cavities predominate in XLRS patient retinas) when injected into areas outside the macula. A preclinical study in non-human primates demonstrated that AAV.SPR promotes transgene expression well beyond subretinal injection bleb margins. This is in stark contrast to benchmark vector AAV5, which remains confined to the original bleb margins. At clinically relevant doses, AAV.SPR efficiently transduces foveal cones without the need for surgical detachment and does not cause inflammation. More information about the study and additional figures are available at: https://atsenatx.com/scientific-approach/laterally-spreading-aav/
“Our novel spreading capsids may be the key to overcoming the challenges associated with intravitreally delivered AAVs in the treatment of XLRS, such as inefficient photoreceptor transduction and inflammation,” said Linda B. Couto, PhD, Chief Scientific Officer of Atsena. “We are excited to continue advancing the research and development of our XLRS program, as we believe our approach facilitates the safe and efficient treatment of the central retina in XLRS patients.”
“We have spent years designing novel AAV capsids for efficient retinal transduction via multiple delivery routes. Our work, as well as that of others, highlights that intravitreally delivered AAVs do not drive sufficient gene expression in photoreceptors to confer therapy, and can even lead to vision-compromising inflammation,” said Shannon Boye, PhD, Founder and Director of Atsena. “AAV.SPR was born out of a desire to drive therapeutic levels of gene expression in photoreceptors while avoiding the surgical risks of foveal detachment, and the immunological complications of intravitreal delivery. The ability to safely and efficiently target foveal cones and to transduce wider expanses of retinal tissue represents a major leap forward in our field.”
