Apellis Announces Detailed Results from Phase 3 DERBY and OAKS Studies
Apellis Pharmaceuticals announced that detailed data from the phase 3 DERBY and OAKS studies were presented for the first time as part of two oral presentations at the Retina Society Annual Scientific Meeting in Chicago.The studies evaluated the efficacy and safety of pegcetacoplan, an investigational, targeted C3 therapy, in geographic atrophy (GA) secondary to age-related macular degeneration (AMD). GA is a leading cause of blindness that impacts more than five million people globally including one million people in the United States.1,2
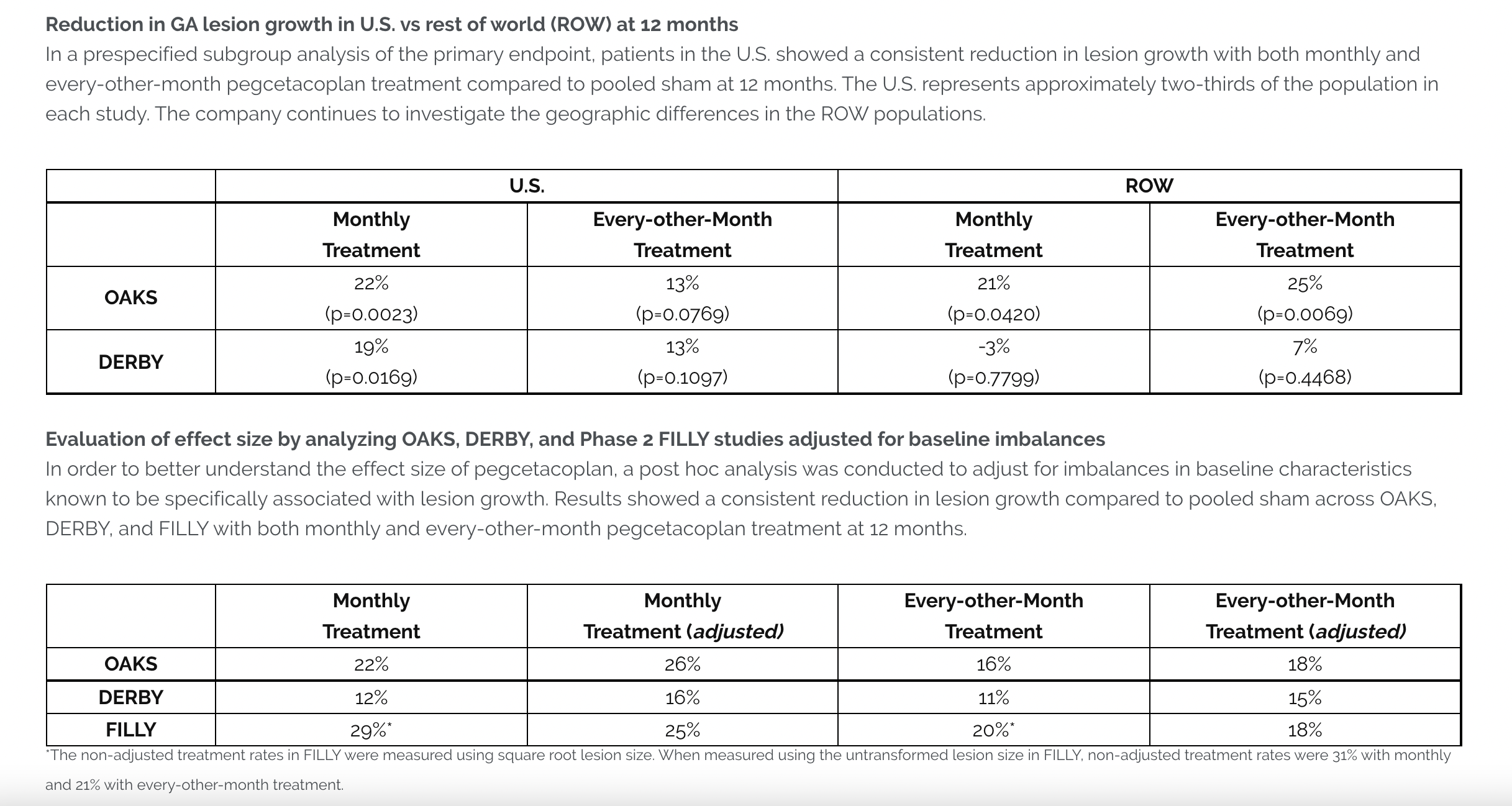
In the OAKS study, monthly (p=0.0003) and every-other-month treatment (p=0.0052) with pegcetacoplan met the primary endpoint, significantly reducing GA lesion growth compared to pooled sham at 12 months. The DERBY study narrowly missed the primary endpoint, showing a reduction in GA lesion growth with monthly (p=0.0528) and every-other-month treatment (p=0.0750) compared to pooled sham at 12 months. In a prespecified analysis of the combined studies, pegcetacoplan showed a reduction in lesion growth in patients with foveal and extrafoveal lesions, with a greater effect in patients with extrafoveal lesions at baseline. Apellis plans to submit a New Drug Application (NDA) for pegcetacoplan for GA to the U.S. Food and Drug Administration (FDA) in the first half of 2022.
“The data presented at Retina Society reinforce that pegcetacoplan is a breakthrough for patients with GA, a relentless disease that leads to blindness and has no treatment,” said Charles Wykoff, M.D., Ph.D., investigator of the OAKS study and director of research, Retina Consultants of Texas. “Pegcetacoplan demonstrated a clinically meaningful treatment effect and favorable safety profile with both monthly and every-other-month dosing, highlighting the potential of pegcetacoplan to become the first treatment for GA.”
The presentations also included new analyses that evaluated the differences between the studies:
Pegcetacoplan demonstrated a favorable safety profile in both Phase 3 studies. The pooled rate of new-onset exudations was 6.0%, 4.1%, and 2.4% in the pegcetacoplan monthly, every-other-month, and sham groups, respectively. Rates of endophthalmitis and intraocular inflammation were generally in line with those reported in studies of other intravitreal therapies.
Detailed efficacy and safety data presented at the Retina Society Annual Scientific Meeting may be accessed on the company’s website.
About DERBY and OAKS
DERBY (621 patients enrolled) and OAKS (637 patients enrolled) are Phase 3, multicenter, randomized, double-masked, sham-controlled studies comparing the efficacy and safety of intravitreal pegcetacoplan with sham injections in patients with geographic atrophy (GA) secondary to age-related macular degeneration (AMD). The primary objective of the studies is to evaluate the efficacy of pegcetacoplan in patients with GA assessed by change in the total area of GA lesions from baseline as measured by fundus autofluorescence at 12 months (p-value less than .05). Patients in DERBY and OAKS will continue on masked treatment for 24 months.
