Identifying and Managing Corneal Dystrophies
AT A GLANCE
- Many patients with a corneal dystrophy (CD) are asymptomatic, but those who do present with symptoms typically report mild to moderate pain, bilateral vision loss of varying degrees, and photophobia.
- Fuchs endothelial CD is the most common type of CD.
- Dystrophies resulting in recurrent corneal erosions require systematic management, which includes artificial tears, viscous nighttime agents, and appropriate use of a bandage contact lens.
Corneal dystrophies (CDs) are a group of inherited corneal diseases that are typically bilateral, symmetric, slowly progressive, and without relationship to environmental or systemic factors.1 The prevalence rate of CDs is reported at 897 per 1,000,000 individuals in the United States.2 Although rare, the effect a CD can have on a patient’s quality of life is significant. As eye care professionals, we must be equipped to properly diagnose and manage these conditions when one presents in clinic.
CDs: THE BASICS
There are more than 20 documented CDs. In 2015, the International Classification of Corneal Dystrophies proposed four subclassifications of CDs based on anatomic location (epithelial/subepithelial, epithelial-stromal, stromal, and endothelial).3 New discoveries and unique case presentations continue to broaden our knowledge and allow further classification of these conditions.
Many patients with a CD are asymptomatic, but those who do present with symptoms typically report mild to moderate pain, bilateral vision loss of varying degrees, and photophobia. They may also experience signs of dry eye, corneal edema, and/or recurrent corneal erosions (RCEs). Treatment ranges widely from artificial tears to corneal transplantation, depending on symptoms and the corneal layer involved.
The following sections provide a brief summary on each of the known CDs grouped into their subclassifications and describe the signs that will help identify the specific type of CD.
EPITHELIAL AND SUBEPITHELIAL DYSTROPHIES
Epithelial Basement Membrane Dystrophy (EBMD)
EBMD is the most common anterior corneal dystrophy (Figure 1). It is also known as anterior basement membrane dystrophy or map-dot-fingerprint dystrophy. EBMD occurs as a result of dysfunctional basal epithelial cells producing an abnormal basement membrane and presents with irregular, gray, map-like patches; intraepithelial cysts; and/or whorl-like fingerprint patterns.3,4

Meesmann Epithelial CD (MECD)
MECD is caused by a mutation in one of the genes responsible for encoding the two cornea-specific cytokeratin units in the epithelium. It is characterized by tiny, gray epithelial vesicles/microcysts that extend to the limbus but are often more dense in the interpalpebral zone. Signs usually appear in infancy or early childhood, but patients remain asymptomatic until middle age.3,4
Epithelial Recurrent Erosion Dystrophy (ERED)
ERED is characterized by RCEs that occur spontaneously or with minimal trauma. RCEs are often severe and can last up to 1 week. Some patients present with resultant subepithelial opacities or keloid-like nodules.3
Gelatinous Drop-Like Dystrophy (GDLD)
GDLD is characterized by subepithelial deposition of amyloid. It initially presents with gelatinous, mulberry-shaped nodules that eventually progress in size, causing stromal opacification and severe vision loss. GDLD is most common in patients of Japanese descent.3,5
Subepithelial Mucinous CD (SMCD)
SMCD presents with subepithelial opacities that are most dense centrally but involve the entire cornea. RCEs occur similarly to those in patients with ERED, but commonly decrease by adolescence. SMCD is quite rare and has only ever been seen in a single three-generation family.3,6
Lisch Epithelial CD (LECD)
LECD, the only known CD with x-chromosomal dominant inheritance, presents with gray opacities arranged in a feathery, whorl-like pattern. Patients often report painless loss of visual acuity, although many are asymptomatic until their sixth decade of life.3,4
EPITHELIAL-STROMAL DYSTROPHIES
Each dystrophy in this category is caused by a mutation in the transforming growth factor beta-induced (TGFBI) gene, which encodes the TGFBI protein. Abnormal TGFBI proteins clump together, causing amyloid deposits in the cornea.
Reis-Buckler CD (RBCD)
RBCD is characterized by fine, reticular corneal opacities that progress to form a honeycomb pattern over the central and midperipheral cornea. Diffuse superficial stromal haze evolves with increased central corneal thickness, irregular astigmatism, RCEs, and decreased corneal sensation.3,7
Thiel-Behke CD (TBCD)
TBCD presents similarly to RBCD, but the signs and symptoms of this CD are milder, causing visual impairment later in life than RBCD. The cornea typically remains regular, vision isn’t affected as severely, and corneal sensitivity is normal.3,7
Lattice CD (LCD)
LCD is characterized by amyloid deposition between the epithelium and Bowman layer, resulting in thin, branching lines in the central cornea. There are two genetically distinct types: LCD type I and LCD type II. LCD type I is a true CD, whereas type II is a systemic condition with ocular manifestations.3,4
Granular CD (GCD)
Each form of this CD—GCD 1 and GCD 2—present with sharply demarcated breadcrumb-like or snowflake-like opacities in the stroma. GCD 2 is distinguished from GDC 1 by the presence of lattice-like deposits, as well as fewer overall deposits. Significantly reduced vision is typically present by the fifth decade of life.3,4
STROMAL DYSTROPHIES
Macular CD (MCD)
MCD presents with poorly defined gray-white opacities located within a hazy stroma, typically resulting in severe vision loss by the fifth decade of life. MCD presents similarly to GCD, but the hazy appearance between opacities is indicative of MCD.3,4
Schnyder CD (SCD)
Within the second decade, SCD presents with subepithelial crystals and/or central corneal haze that eventually progresses to a ring-like pattern. By the third decade, arcus is noted (Figure 2). Patients with advanced SCD may present with glare, decreased corneal sensitivity, and reduced vision.3,8

Congenital Stromal CD (CSCD)
CSCD is characterized by diffuse clouding of the cornea with white flake-like stromal deposits. The presentation can be severe enough to cause significant vision loss. CSCD has only been reported in five families.3,4
Fleck CD (FCD)
Although most patients are asymptomatic, FCD presents with small, gray-white, dandruff-like flecks scattered sparsely throughout the stroma. FCD is nonprogressive and does not typically affect vision.3,4
Posterior Amorphous CD (PACD)
PACD is characterized by gray-white, sheet-like opacities scattered in the central and peripheral stroma. Typically, PACD is nonprogressive and has minimal effect on vision.3,4
Central Cloudy Dystrophy of Francois (CCDF)
CCDF closely resembles crocodile shagreen, with lizard-like, gray, polygonal stromal opacities. As most patients are asymptomatic and CCDF is nonprogressive, treatment is rarely required.3,4
Pre-Descemet CD (PDCD)
PDCD presents with focal, fine, gray opacities made of lipids located in the deep stroma, immediately anterior to Descemet membrane. Most patients are asymptomatic.3,4
ENDOTHELIAL DYSTROPHIES
Posterior Polymorphous CD (PPCD)
PPCD can present with a variety of corneal lesions that can be nodular, vesicular, or blister-like in shape and/or linear lesions resembling railroad tracks. Keratoconus and corneal edema can be associated with PPCD, leading to significant vision loss.3,4
Congenital Hereditary Endothelial Dystrophy (CHED)
CHED is characterized by diffuse corneal edema, varying from a bluish, ground glass appearance to complete opacification. Due to the edema, a significant increase in corneal thickness occurs along with reduced vision.3,4
X-Linked Endothelial CD (XECD)
In male patients, XECD presents with corneal opacification ranging from a milky, ground glass appearance to diffuse corneal haze similar to CHED, moon crater-like changes in the endothelium, and subepithelial band keratopathy. Female patients with XECD present with only the moon crater-like endothelial abnormalities.3,9
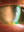
Fuchs Endothelial CD (FECD)
FECD is the most common type of CD (Figure 3). Initially, FECD presents with guttata along Descemet membrane located in the central cornea. In more advanced FECD, corneal edema causes haze, endothelial folds, epithelial microcysts, epithelial bullae, and eventually, subepithelial fibrosis.3,10
MANAGEMENT
Management of a CD largely depends on the patient’s symptoms. Dystrophies resulting in RCEs require systematic management, which includes artificial tears, viscous nighttime agents, and appropriate use of a bandage contact lens. For severe RCEs, anterior stromal puncture, superficial keratectomy, and/or phototherapeutic keratectomy may be warranted. Topical NSAIDs may be used for pain management.
Amniotic membranes should be considered as an alternative to a traditional bandage contact lens for the added therapeutic benefits of increasing healing time and reducing inflammation and scarring. Scleral lenses are another option for improving the vision of patients who present with irregular astigmatism and/or corneal scarring (Figure 4).

For severe manifestations of CDs, a corneal transplant is often the best option to reduce symptoms and improve visual acuity. A deep anterior lamellar keratoplasty (DALK) involves removing the epithelium, Bowman layer, and as much of the stroma as possible, while leaving the endothelium and Descemet membrane intact. A DALK is ideal for dystrophies in which the anterior cornea is affected but the health of the posterior cornea is normal.
To treat endothelial dystrophies, Descemet stripping endothelial keratoplasty (DSEK) or Descemet membrane endothelial keratoplasty (DMEK) is warranted. A DSEK involves removing diseased endothelium and Descemet membrane and replacing them with donor tissue, whereas a DMEK is purely a transplant of the endothelium.
GENETIC TESTING CAN HELP
Due to the similar presentations of many of the CDs reviewed here, differentiating between them is often challenging. Start by narrowing the patient’s suspected CD down into one of the four subclassifications, then see if you can make any further determinations based on signs and symptoms. When in doubt, genetic testing may be warranted to confirm the diagnosis.
Ready to Claim Your Credits?
You have attempts to pass this post-test. Take your time and review carefully before submitting.
Good luck!
Recommended
- Cornea/Anterior Segment
Epi-Off Versus Epi-On: the Conversation Continues
Mitch Ibach, OD, FAAO; Sam Rivet, ODMitch Ibach, OD, FAAO; Sam Rivet, OD



