Notal Vision: Clinical Trials Demonstrate Value of Home OCT

Notal Vision reported that the latest data on its investigational home-based optical coherence tomography (OCT) and how the service can be used to manage wet age-related macular degeneration (AMD) were recently presented at the Angiogenesis, Exudation, and Degeneration and Macula Society 2023 meetings. Notal Vision is developing technology designed to provide patient-initiated retinal OCT scans to support the management of patients with wet AMD, complementing existing standard of care treatments as well as emerging longer acting drugs and drug delivery systems.
A presentation by Nancy Holekamp, MD, Pepose Vision Institute, reviewed several clinical trials that show the progress toward implementing home OCT in the management of patients with wet AMD. With participation of over 200 subjects and 8 retina specialists, the trials assessed the usability of the device by patients, demonstrated extended use of the device at home by patients, the interpretation of the home OCT images by a reading center, and driving patient management with AI- based notifications and physician review of home OCT data under IRB approved protocol.
“The experience with home OCT has given me an understanding of the value of missing information in between the office visits,” Dr. Holekamp said in a company news release. “I believe the technology will have a twofold impact: individualizing the care of patients and better understanding the dynamics of AMD.”
The Notal Vision Home OCT incorporates a deep learning-based algorithm, called Notal OCT Analyzer (NOA), for automatic quantification of retinal fluid, a key biomarker in wet AMD treatment efficacy, which provides physicians with additional information not currently provided by the standard of care clinical evaluations. Presentations from Anat Loewenstein, MD, professor and director of the Department of Ophthalmology at Tel Aviv Medical Center in Tel Aviv, Israel, provided further insights into data generated by near daily self-imaging and quantification of home OCT derived retinal fluid volumes. She reviewed the characteristics of disease reactivation, treatment responses, and demonstrated heterogeneity across the studied patients.
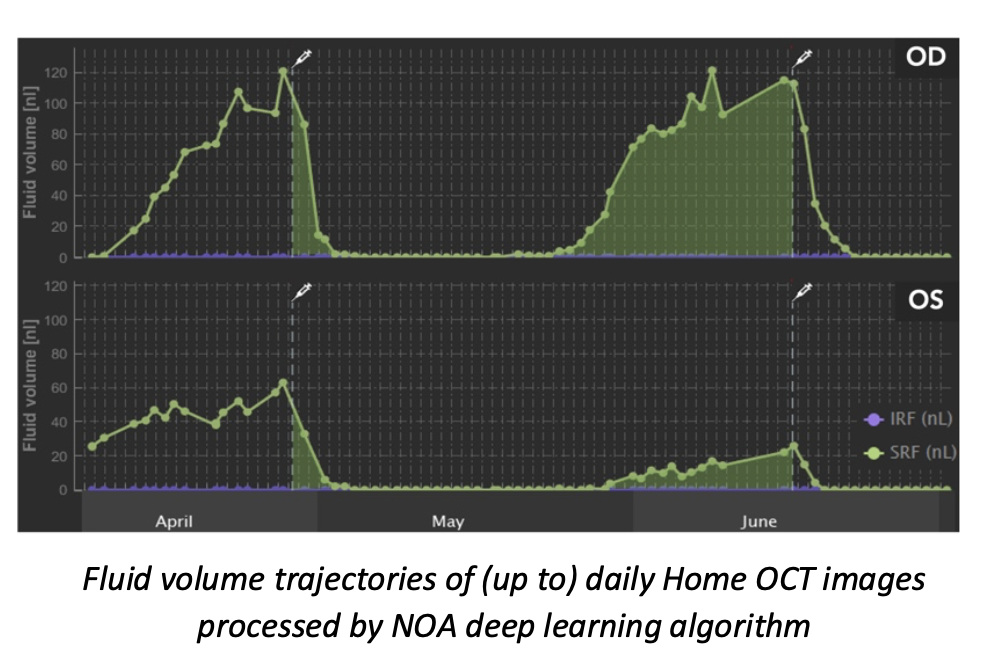
“The purpose was to evaluate the effect of timely treatment and the implications and benefits of incorporating home OCT into patient care,” said Prof. Loewenstein. “I think home OCT will change significantly the way we manage the patient.”
Dinah Zur, MD from the Tel Aviv Medical Center also reviewed the use of NOA in analyzing in-office OCT images of 4,485 eyes from 3,637 patients in the BIRAX project with the University of Belfast and Tel Aviv Medical Center, which aims to provide measurable indicators to enable models for personalized treatment approaches in wet AMD.
Home OCT has received a “Breakthrough Device” designation by the FDA. Designated reimbursement codes for the Notal Vision Monitoring Center’s device supply and monitoring services, as well as physician monthly report review, have already been established with support by the American Academy of Ophthalmology (AAO). Notal Vision looks forward to releasing Home OCT in Q4 2023, following an FDA premarket review, to US-based clinics that partner with its Medicare-accredited Monitoring Center through patient referral.
“The studies presented at the Angiogenesis and Macula Society meetings show critical milestones in bringing home OCT to market and expanding our digital health offerings in ophthalmology,” said Kester Nahen, PhD, CEO of Notal Vision.
