GenSight Biologics Confirms Sustained Efficacy and Safety of Bilateral Lumevoq Injections at 2-Year Follow-Up of REFLECT Phase 3 Trial
GenSight Biologics reported topline efficacy and safety results at 2 years post-treatment administration in the REFLECT Phase 3 clinical trial with Lumevoq. The results show sustained efficacy and safety for bilateral intravitreal injection of the gene therapy, including better efficacy compared to unilateral injection.
The findings reinforce the results observed at 1.5 years post-treatment administration, which were reported in June 2021.
“The REFLECT trial’s demonstration of a sustained, significant and safe improvement in visual acuity for LHON patients treated bilaterally with Lumevoq provides additional impetus for our push to gain regulatory approval,” Bernard Gilly, CEO and Co-Founder of GenSight Biologics, said in a company news release. “Patients afflicted with LHON who are losing their sight deserve access to a treatment like Lumevoq.”
Designed under a Special Protocol Assessment with the FDA, REFLECT is a randomized, double-masked, placebo-controlled phase 3 trial involving 98 subjects with vision loss due to Leber Hereditary Optic Neuropathy (LHON) caused by a mutated ND4 mitochondrial gene; enrolled ND4 subjects had vision loss up to 1 year from onset. The ND4 mitochondrial mutation is associated with the most severe clinical form of LHON, with poor overall visual outcomes.1 All subjects received an intravitreal injection (IVT) of Lumevoq in their first affected eye. The second affected eye was randomized to either a second IVT of Lumevoq or a placebo IVT, which was administered on the same day or the following day. 48 subjects were randomized to Lumevoq bilateral treatment, and 50 to Lumevoq unilateral treatment (first-affected eye treated with Lumevoq, second-affected eye treated with placebo).
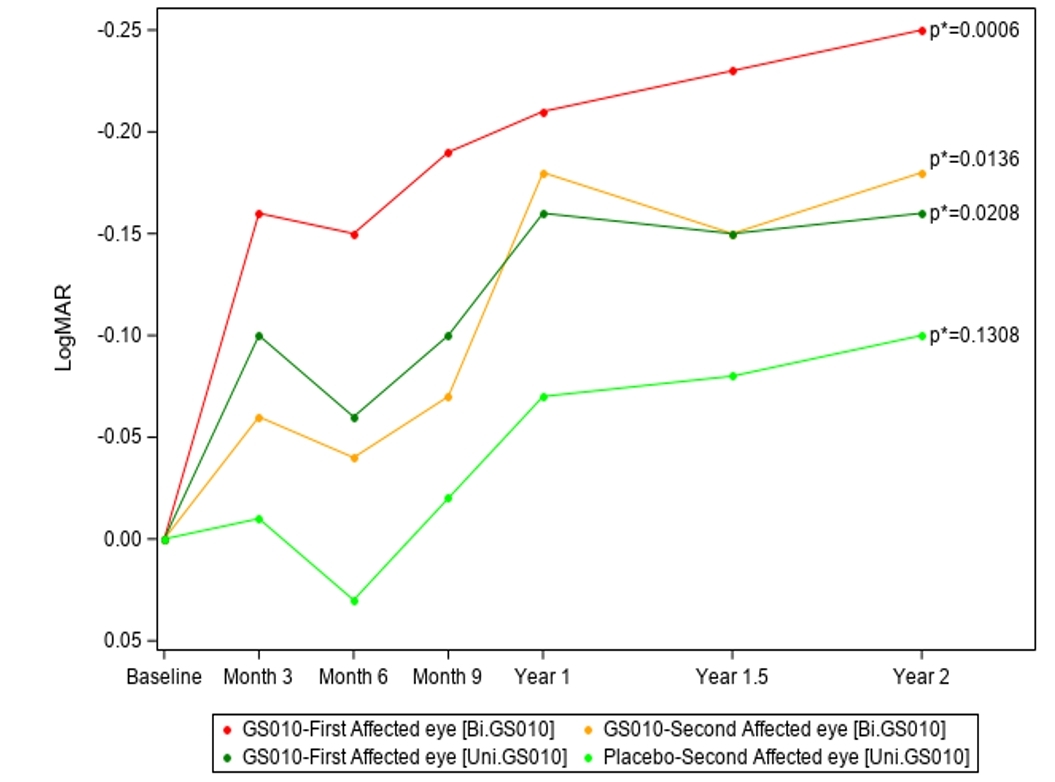
Significant visual acuity improvement over baseline, with better results for bilaterally injected patients
Two years after injection, the mean best-corrected visual acuity (BCVA) in Lumevoq-treated eyes was statistically significantly better than baseline, whereas the improvement from baseline was not statistically significant in placebo-treated eyes. The results indicate a sustained treatment effect for all subjects, with the improvement being greater among bilaterally treated patients.
Table 1: Change in Best-Corrected Visual Acuity (BCVA) versus Baseline, 2 Years after Injection
1st affected eye | 2nd affected eye | |||
Subjects bilaterally | LUMEVOQ® | LUMEVOQ® | ||
Subjects unilaterally | LUMEVOQ® | PLACEBO |
The contralateral effect observed with placebo at 2 years is consistent with that which was documented in sham-treated eyes in the REVERSE2 and RESCUE3 trials.
Year 2 analyses also confirm the dose effect that was noted at Year 1.5: the mean BCVA at 2 years for bilaterally and unilaterally treated subjects reached 1.32 and 1.44 LogMAR, respectively, with an absolute difference between arms of +6 ETDRS letters in favor of bilaterally treated subjects.
Responder analyses point to the benefits of treatment for patients that would otherwise have experienced significant vision loss with a very low likelihood of spontaneous recovery.1 For example, 60% of the bilaterally treated patients (56% of unilaterally treated patients) who had vision above the threshold of legal blindness in at least one eye remained above the threshold at Year 2.
Efficacy demonstrated even more clearly in visual acuity improvement from nadir
Comparison against nadir (i.e., the worst BCVA recorded from baseline to Year 2) more starkly demonstrates the efficacy of Lumevoq, even for the placebo eyes that improved via a contralateral treatment effect.
Table 2: Change in Best-Corrected Visual Acuity (BCVA) versus Nadir, 2 Years after Injection
1st affected eye | 2nd affected eye | |||
Subjects bilaterally | LUMEVOQ® | LUMEVOQ® | ||
Subjects unilaterally | LUMEVOQ® | PLACEBO |
Responder analyses indicate that the treatment effect is not limited to just a minority of subjects. Two years after injection, 73% of bilaterally treated subjects and 66% of unilaterally treated subjects had experienced a clinically meaningful improvement of at least -0.3 LogMAR (+15 ETDRS letters) relative to their observed nadir.
Table 3: Responder Analyses, Based on Change from Nadir at Year 2
Definition of Responder | -0.3 LogMAR improvement in | Clinically Relevant Recovery* | ||
Subjects bilaterally | 73% | 75% | ||
Subjects unilaterally | 66% | 60% |
Note: *“Clinically Relevant Recovery” is defined as: i) For eyes on-chart at nadir, an improvement of ≤ -0.2 LogMAR (≥10 ETDRS letters) from nadir; or ii) For eyes off-chart at nadir, eyes which became on-chart (i.e., BCVA ≤1.6 LogMAR).
Bilateral injections have a favorable safety profile
The favorable safety profile of Lumevoq was confirmed. There was no study discontinuation related to systemic or ocular adverse events, and there were no serious ocular adverse events. The main ocular adverse event was intraocular inflammation, which was mostly mild and responsive to conventional treatment. The favorable safety profile was comparable in unilaterally and bilaterally treated subjects.
“The persistence of Lumevoq efficacy is remarkably consistent across the development program, so that the REFLECT results bolster the evidence provided by 3 years of data from RESTORE4 and 5 years of data from REVEAL,” noted Magali Taiel, MD, Chief Medical Officer of GenSight Biologics.
Results of the 4-year follow-up of RESTORE are expected to be available in January 2022.
Dr. Taiel added, “Moreover, we affirm the insight that bilateral injection of Lumevoq is the best option for patients with ND4 Leber Hereditary Optic Neuropathy.”
REFLECT patients have been invited to participate in a long-term follow-up that will monitor the safety and efficacy of Lumevoq up to 5 years post-injection.
References:
- Newman NJ, Carelli V, Taiel M, Yu-Wai-Man P. Visual outcomes in Leber hereditary optic neuropathy subjects with the m.11778G>A (MTND4) mitochondrial dna mutation. J Neuroophthalmol. (2020) 40:547–57. doi: 10.1097/WNO.0000000000001045.
- Yu-Wai-Man P, Newman NJ, Carelli V, Moster ML, Biousse V, Sadun AA, et al. Bilateral visual improvement with unilateral gene therapy injection for Leber hereditary optic neuropathy. Sci Transl Med. (2020) 12:eaaz7423. doi: 10.1126/scitranslmed.aaz7423
- Newman NJ, Yu-Wai-Man P, Carelli V, Moster ML, Biousse V, Vignal-Clermont C, et al. Efficacy and safety of intravitreal gene therapy for Leber hereditary optic neuropathy treated within 6 months of disease onset. Ophthalmology. (2021) 128:649–60. doi: 10.1016/j.ophtha.2020.12.012.
- Biousse V, Newman NJ, Yu-Wai-Man P, Carelli V, Moster ML, Vignal-Clermont C, et al. Long-term follow-up after unilateral intravitreal gene therapy for Leber hereditary optic neuropathy: The RESTORE study. J Neuroophthalmol. (2021) 41:309-315.
