GATHER2 Study Shows Izervay Monthly or Every Other Month Reduced GA Lesion Growth Through 2 Years

Astellas Pharma announced results from the GATHER2 phase 3 clinical trial, demonstrating Izervay (avacincaptad pegol intravitreal solution) continued to reduce the rate of geographic atrophy (GA) lesion growth for both every month (EM) and every-other-month (EOM) dosing vs. sham through 2 years of treatment in patients with GA secondary to age-related macular degeneration (AMD). The GA treatment benefit with Izervay vs. sham was observed as early as 6 months, continued to increase over time through 2 years, and more than doubled over 2 years compared to year 1. These findings were presented during the American Academy of Ophthalmology 2023 Annual Meeting in San Francisco.
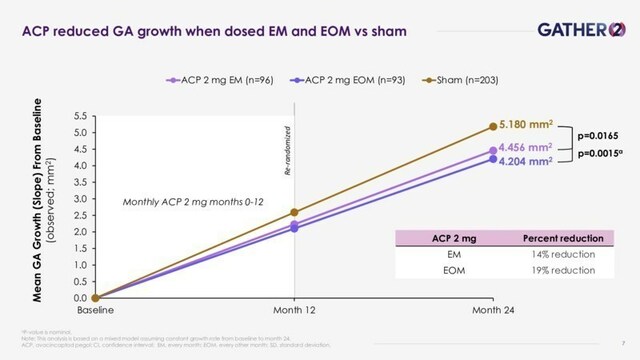
Key study results include:
- Izervay dosed EM through 2 years demonstrated a statistically significant year-over-year reduction of 14% in the mean rate of GA growth at 2 years from baseline vs. sham (P=0.0165), the primary objective of the year 2 analyses from GATHER2.
- EOM dosing of Izervay, after a year of monthly dosing, resulted in a reduction of 19% in the mean GA growth rate at 2 years vs. sham (nominal P-value=0.0015).
- Izervay treatment effect more than doubled over 2 years compared to 1 year.
- The prespecified objective of demonstrating that Izervay reduced the rate of ≥15-letter persistent vision loss compared to sham over 2 years was not statistically significant. Persistent vision loss will be further explored across several sensitivity analyses.
- Izervay was well tolerated over 2 years, with one case each of non-serious intraocular inflammation (IOI) and culture-positive endophthalmitis. There were no cases of ischemic neuropathy or retinal vasculitis.
- Over 2 years, only a slight increased incidence of choroidal neovascularization (CNV) was observed for Izervay pooled vs. sham. In year 2, the incidence of CNV was similar for Izervay EOM vs. sham.
"GA is a debilitating, progressive disease that can impact patients' independence. These exciting results demonstrate year-over-year reductions in the rate of GA lesion growth in patients treated with monthly and every-other-month dosing compared to sham," Arshad M. Khanani, MD, MA, FASRS, Director of Clinical Research at Sierra Eye Associates, Reno, Nevada, said in a company news release. "The GA treatment benefit was observed as early as 6 months and continued to increase over time. The safety profile over 2 years was consistent with year 1, with no new safety signals identified. These results further validate that Izervay is an effective and safe treatment option for patients with GA."
Treatment-emergent adverse events (TEAEs) over 2 years were similar and consistent with year 1. Ocular TEAEs were observed in 64.0% of eyes in the Izervay treatment group and 48.2% in the sham group.
- The three most common ocular TEAEs in patients treated with Izervay vs. sham over 2 years were conjunctival hemorrhage (16.9% vs. 9.0%), intraocular pressure increased (13.3% vs. 0.9%) and CNV (11.6% vs. 9.0%).
- Serious ocular TEAEs over 2 years were equivalent between the Izervay and sham study groups (1.8% vs. 0.9%). In year 2, there was one case of culture-positive endophthalmitis and one case of subluxated intraocular lens for Izervay compared to no such cases in the sham group.
Izervay was approved by the FDA on August 4, 2023, for the treatment of GA secondary to AMD and is currently under review by the European Medicines Agency.
Astellas has already reflected the impact from this result in its financial forecast of the current fiscal year ending March 31, 2024.
About the GATHER2 Clinical Trial
GATHER2 (NCT04435366) was a randomized, double-masked, sham-controlled, multicenter phase 3 clinical trial to evaluate the safety and efficacy of intravitreal administration of avacincaptad pegol (ACP) in 448 enrolled patients with GA secondary to AMD. ACP met its primary objective at 12 months, for which patients were randomized to receive either ACP or sham procedure monthly. In year 2 of the study, patients treated with ACP in year 1 were re-randomized to receive either ACP dosed monthly (EM, n=96) or every other month (EOM, n=93); patients who received sham in year 1 continued to receive sham in year 2 (n=203). IZERVAY is continuing to be evaluated in an open-label extension study.
