Bausch + Lomb Publishes Phase 4 Data on the Early Effects of Miebo for Dry Eye Disease

Bausch + Lomb announced that Ophthalmology and Therapy has published results from a phase 4 study which assessed early patient-reported outcomes with Miebo in patients with dry eye disease (DED). Miebo, indicated for the signs and symptoms of DED, is the first and only prescription treatment to directly target tear evaporation, according to B+L.
The study examined the results of Miebo after the first use and during the first 2 weeks of treatment. These data build on results from the GOBI and MOJAVE pivotal studies in which patients experienced significant improvement in the signs and symptoms of DED as early as day 15, with continued improvement through day 57.
The prospective, multicenter, open-label phase 4 study evaluated the effect of Miebo on symptom severity and frequency early in treatment. Inclusion criteria aligned with the phase 3 studies where all patients had a history of dry eye disease and evidence of meibomian gland dysfunction. Patients completed early outcome surveys during four clinic visits (day 1 [pretreatment; 5 and 60 minutes post-first administration] and days 3, 7, and 14). Patients rated symptom severity, symptom frequency and treatment satisfaction on a visual analog scale (VAS) from 0 to 100.
Key points from the trial (for all results P<0.0001):
- Patients reported that Miebo significantly reduced overall symptom severity at the primary endpoint of day 7.
- The primary endpoint of change from baseline in the severity of overall dry eye symptoms at day 7 was met. Mean overall symptom severity decreased significantly from 72.1 (17.0) at baseline to 27.8 (22.3) at day 7 (mean change, − 44.5).
- Significant symptom relief was observed within 5 and 60 minutes after a single administration on day 1:
- The mean score on the VAS for overall dry eye symptoms was 72.1 (17.0) at baseline and decreased to 38.5 (22.8) at 5 min post-administration and 31.7 (22.1) at 60 min post-administration.
- Significant reductions were seen for overall symptom severity and across all symptoms, including dryness, blurred vision, eye irritation, light sensitivity, eye tiredness, burning/stinging, itching and eye pain, at all post-baseline assessments.
- Significant decreases were also observed in mean frequency of awareness of dry eye symptoms, fluctuations in quality of vision and time experiencing the most bothersome symptoms:
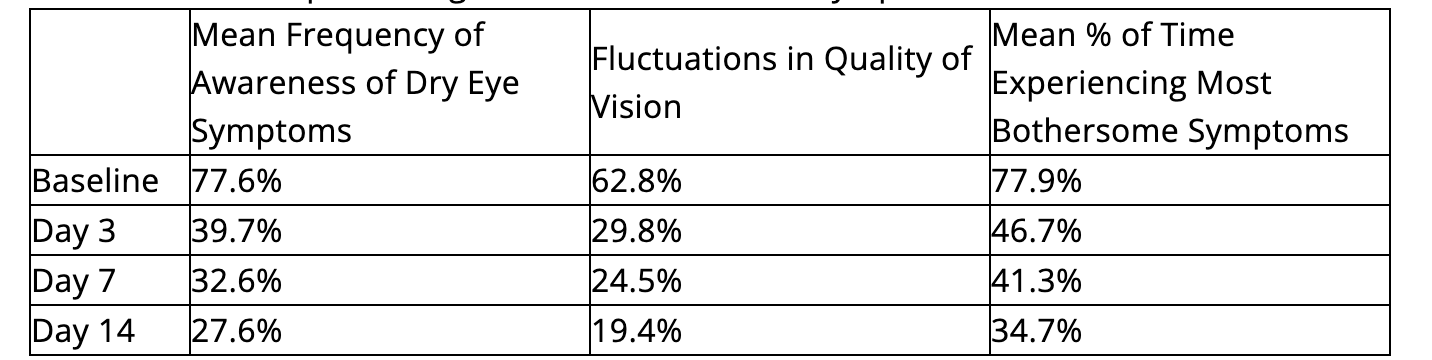
- Median satisfaction ratings increased steadily from 83.0 at day 3, 86.0 at day 7, and 90.0 at day 14, reflecting high patient satisfaction with Miebo.
- From 10 descriptors, study participants most commonly chose “silky, smooth and soothing” to describe how the drop felt on administration.
- Miebo was well tolerated with no reports of treatment-related adverse events.
