Aldeyra Therapeutics Achieves Primary Endpoints in Dry Eye Disease Chamber Crossover Clinical Trial

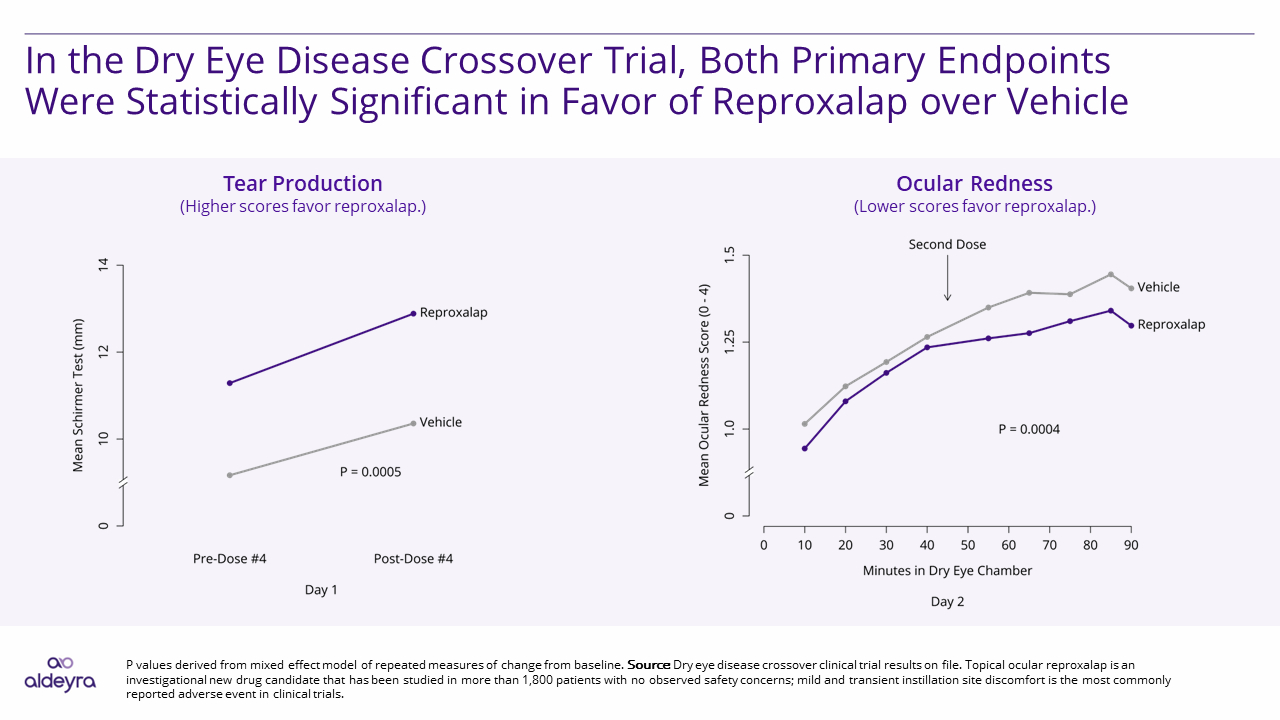
Aldeyra Therapeutics announced the achievement of the primary endpoints in a sequence-randomized, double-masked, vehicle-controlled crossover clinical trial of 0.25% reproxalap ophthalmic solution, an investigational new drug candidate, for the treatment of dry eye disease. Reproxalap was statistically superior to vehicle for each of the two prespecified primary endpoints, ocular redness in a dry eye chamber (P=0.0004) and Schirmer test (P=0.0005), a measure of tear production, after a single day of dosing. The secondary endpoint of Schirmer test ≥10 mm responder analysis, which was multiplicity-controlled, was also achieved (P=0.0361).
“Statistical significance in favor of reproxalap over vehicle for all three dry eye disease signs that we intend to submit to a new drug application, in addition to observed rapid symptomatic improvement, favorably positions reproxalap, if approved for sale, as a potentially differentiated therapeutic option for the treatment of dry eye disease,” Todd C. Brady, MD, PhD, President and Chief Executive Officer of Aldeyra, said in a company news release.
Relative to vehicle, statistically significant reduction in ocular redness was observed following treatment with reproxalap as soon as 10 minutes after dry eye chamber entry, which was the first time point assessed, and over the majority of chamber assessment time points, including the final time point 90 minutes after chamber entry. Schirmer test, which was assessed approximately 10 minutes before and after the fourth dose of reproxalap or vehicle, was statistically significant in favor of reproxalap at both time points, indicating the potential activity of prior dosing as well as additional activity of the fourth dose over a single day of drug administration. The Schirmer test ≥10 mm responder analysis has been reported to correlate with symptomatic improvement in dry eye disease,1 and achievement of the responder endpoint is consistent with the symptomatic improvement observed in the crossover clinical trial: reproxalap was statistically lower than vehicle for the secondary endpoints of ocular dryness (P=0.0068), discomfort (P<0.0001), grittiness (P=0.0001), stinging (P=0.0001), burning (P<0.0001), and itching (P=0.0003) symptoms.
No safety signals were observed in the trial, and reproxalap was well tolerated; there were no treatment-emergent moderate or serious adverse events. Three patients discontinued due to adverse events, two during vehicle administration and one during reproxalap administration. Reproxalap has now been studied in over 1,800 patients. The most common reported adverse event in clinical trials is mild and transient instillation site discomfort.
Pending discussion with the FDA, Aldeyra intends to submit the crossover clinical trial, which was designed to be adequate and well-controlled, as part of a dry eye disease new drug application (NDA) for reproxalap, including data on ocular redness, Schirmer test, and the Schirmer test ≥10 mm responder analysis. A pre-NDA meeting with the FDA has been scheduled for the third quarter of 2022. The NDA submission is expected to include a comprehensive set of data from acute and chronic clinical trials, as well as a combination of challenge and field-based assessments and parallel-group and crossover clinical trial designs.
“With another set of positive clinical results showing improvement in both the signs and symptoms of dry eye disease, I believe that reproxalap is an eagerly anticipated advancement for our patients suffering from dry eye disease because available therapies are often inadequate,” John P. Berdahl, MD, a specialist in diseases of the anterior segment at Vance Thompson Vision and Professor of Ophthalmology at the Sanford School of Medicine, said in a company news release.
