Aldeyra Achieves Primary Endpoint in Phase 3 Dry Eye Disease Clinical Trial of Reproxalap

Aldeyra Therapeutics announced the primary endpoint has been achieved in its phase 3 trial of investigational reproxalap for dry eye disease.
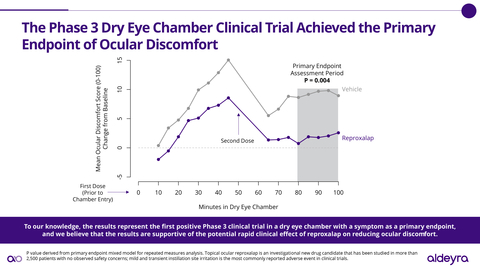
In the randomized, double-masked, vehicle-controlled dry eye chamber clinical trial, reproxalap 0.25% was statistically superior to vehicle for the prespecified primary endpoint of ocular discomfort (P=0.004).
“To our knowledge, the results announced today represent the first positive phase 3 clinical trial in a dry eye chamber with a symptom as a primary endpoint, and we believe that the results are supportive of the potential rapid clinical effect of reproxalap on reducing the ocular discomfort associated with dry eye disease,” Todd C. Brady, MD, PhD, President and Chief Executive Officer of Aldeyra, said in a company news release.
In November 2023, the FDA issued a complete response letter to Aldeyra's new drug application (NDA) submission for reproxalap. No safety or manufacturing issues with reproxalap were identified, however, the FDA letter stated that the NDA did not demonstrate “efficacy in treating ocular symptoms associated with dry eyes” and that “at least one additional adequate and well-controlled study to demonstrate a positive effect on the treatment of ocular symptoms of dry eye” should be conducted.
In the phase 3 clinical trial, patients were administered vehicle before and during exposure to a dry eye chamber in a manner that Aldeyra said it believes is consistent with the FDA’s dry eye disease draft guidance.[1] Qualifying patients were subsequently randomized to receive either reproxalap or vehicle before and during exposure to an additional dry eye chamber. Of the 132 patients randomized, 66 patients received reproxalap and 66 patients received vehicle. The primary endpoint was ocular discomfort from 80 to 100 minutes in the chamber. The dry eye chamber clinical trial was designed to satisfy the FDA’s NDA resubmission requirement, identified in the previously received complete response letter, of “at least one additional adequate and well-controlled study to demonstrate a positive effect on the treatment of ocular symptoms of dry eye.” Through the FDA Special Protocol Assessment process and additional comments, the FDA provided feedback on the clinical trial protocol and statistical plan.
"To Aldeyra’s knowledge, in patients with dry eye disease, reproxalap is the first investigational drug with pivotal data supportive of acute and chronic activity in reducing symptoms, and the first investigational drug for chronic administration with pivotal data supportive of acute activity in reducing ocular redness," the company stated in a news release.
There were no safety signals observed in the clinical trial, and reproxalap was observed to be well tolerated. Consistent with prior clinical trials, the most commonly reported adverse event was mild and transient instillation site discomfort. No treatment-related discontinuations were reported. Reproxalap has now been studied in over 2,500 patients.
The potential NDA resubmission is anticipated in 2024, according to Aldeyra. Based on FDA guidance, the resubmission NDA review period is expected to be 6 months.