Abionyx Pharma Announces Its Strategy in Ophthalmology

France-based Abionyx Pharma announced announced its strategy in ophthalmology and new positive preclinical results in two technological platforms: apotherapy and biovectorisation.
"The acquisition of IRIS Pharma, one of the world leaders in contract research in ophthalmology, a little over a year ago, has borne fruit and has enabled us to structure a value-creating strategy based on our flagship asset, the only natural recombinant apoA-I lipoprotein in the world and one of the most advanced biomedicines," Cyrille Tupin, Chief Executive Officer of Abionyx Pharma, said in a company news release. "Indeed, studies with IRIS Pharma have determined the deployment of a strategy based on two technological platforms: apotherapy that means an innovative therapy based on our natural recombinant apoA-I alone, and biovectorization meaning incorporation of various active ingredients into our proprietary apoA-I complex used as a vector. The preclinical results of our biovector in ophthalmology are very promising, especially for corticoids, in order to optimize their efficacy while limiting their side effects, which are one of the major concerns in ophthalmology today worldwide."
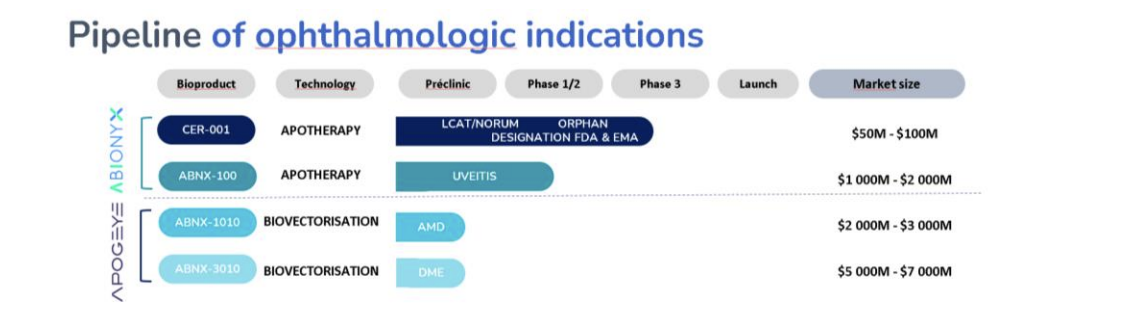
Abionyx Pharma is developing two technological platforms: apotherapy only based on the apoA-I, and biovectorization using apoA-I complexes as a vector to deliver several active ingredients. These two platforms have multiple possible applications in ophthalmology, according to Abionyx. The company has chosen to focus its apotherapy approach initially on the ultra-rare LCAT disease, known as Norum's disease, and on uveitis. Abionyx Pharma's drug candidates for LCAT and uveitis, CER-001 and ABNX100, respectively, are intended to be administered as systemic intravenous injections to target patients with corneal opacity or ocular inflammation in the setting of uveitis to achieve significant functional visual improvement.
Abionyx Pharma's most advanced drug candidate in ophthalmology, CER-001, targeting Fish-Eye Disease in LCAT Deficiency, is currently being used under compassionate approval in Europe. CER-001 has been granted Orphan Drug status in Europe by the EMA and in the United States by the FDA. ABNX-100 in uveitis will enter the clinical phase as soon as the company has received regulatory approval for the advancement of sepsis, as its systemic treatment is very similar to the targeted treatment in uveitis in apotherapy.
Abionyx's recombinant natural apoA-I complexes have been shown to be effective in resolving systemic inflammation as demonstrated in the phase 2 RACERS study, in addition to offering a reparative action on epithelial cells. As far as biovectorization is concerned, uveitis can be treated, but two other major ophthalmology indications are targeted, namely age-related macular degeneration (AMD) and diabetic macular edema (DME) through two other drug candidates, ABNX-1010 and ABNX-3010.
New pipeline of ophthalmologic indications. Based on this new strategy, ABIONYX Pharma communicates a new and more precise pipeline of indications.