Jessilin Quint, OD, MBA, FAAO (Moderator): We’ve gathered as optometric colleagues to discuss our management strategies for dry eye disease (DED). Thanks to an abundance of research over the past several years, we’ve learned that DED is a complex, multifactorial process that can be symptomatic or asymptomatic. It can have signs and symptoms that don’t correlate. It can involve meibomian gland dysfunction, evaporation, aqueous deficiency, or all of these. In addition, inflammation can be present regardless of etiology.
DED is widespread in the US. In 2023, Bausch + Lomb commissioned the Harris Poll to conduct a 1-year poll among adults in the US to understand the prevalence of and perceptions around DED and eye dryness. Harris polled 2,003 adults, 461 of whom were “DED sufferers” (defined as people who often/always experience eye dryness or had been diagnosed by a healthcare professional with dry eye disease) and 1,542 of whom were non-sufferers. Approximately 24% of US adults are diagnosed with DED and/or regularly experience ocular dryness, and 14% of respondants answered that they experience ocular dryness “often, always, or almost always” (Figure 1).1
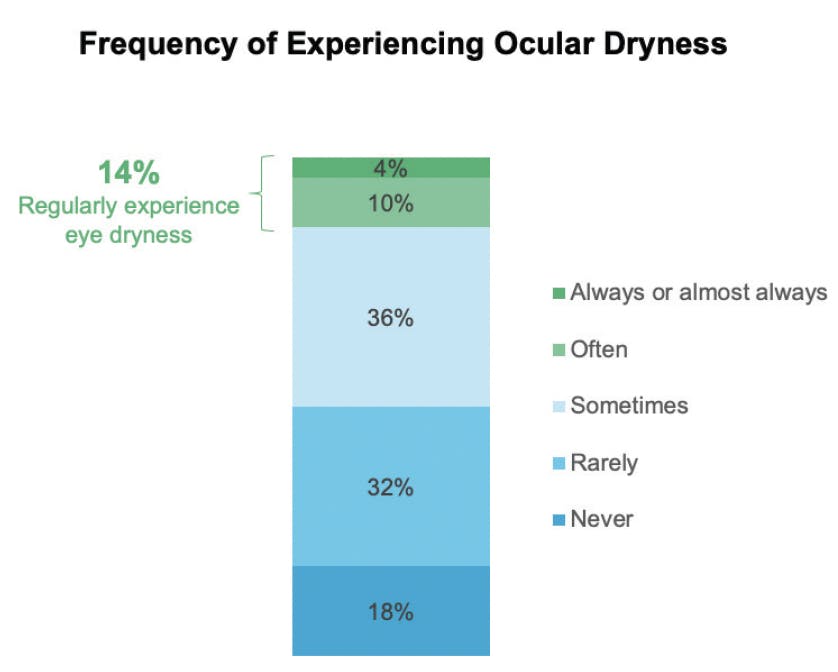
Figure 1. A Harris poll conducted from April 2023 through April 2024 was sent to 2003 adults in the US. Of the respondants, 14% said that they experience symptoms “always, almost always, or often.” (Data on file, Bausch + Lomb)
Fortunately, we eye care practitioners (ECPs) now have the availability of targeted prescription therapies to treat these varying presentations of DED. As a profession, we continue to educate our patients that over-the-counter (OTC) artificial tears are not the only treatment we have for their ocular symptoms, and those symptoms are not something to ignore, especially if they persist.
IDENTIFYING DED IN CLINICAL PRACTICE
Dr. Quint: Let’s begin by each describing how we identify DED in our practices. How many of our new patients are diagnosed with DED versus undiagnosed?
Dr. Schweitzer: I see two main types of DED patients: those who have a DED diagnosis and are being treated, and those who have a diagnosis but are not being treated. Furthermore, I estimate that 65% to 70% of the presurgical patients in my practice have undiagnosed DED. I prefer a proactive management approach, because the data tell me that DED will become a bigger problem if left untreated.
Dr. Dierker: I think we have to screen for both symptoms and signs of DED. We also need to educate patients that symptoms like fluctuating vision could be DED, and about the necessity of treating those symptoms. We must identify all preoperative patients who have visually significant dry eye. I diagnose DED in new patients all day, every day, according to the DEWS II definition: a symptomatic patient who has signs of loss of homeostasis of the tear film, be it osmolarity; corneal, conjunctival, or lid-margin staining; or reduced tear breakup time (TBUT).2
Dr. Davidson: Like Dr. Schweitzer, most of my patients who present for full examinations and show signs of DED are unaware they have it. Many primary care eye doctors are mainly focused on refractive issues (glasses and contacts) and their patients’ chief complaints. These colleagues need to understand that dry eye is a refractive issue. It can cause visual fluctuations.3
Dr. Quint: It’s an important point that symptoms of DED can have such a visual impact when a patient is behind a phoropter, and it’s our job to listen for those clues of whether blinking clears the patient’s vision. We don’t necessarily need fancy diagnostic equipment to diagnose DED.
Dr. Dierker: We should always ask the patient what their treatment goal is. If they say, “I want to see better,” or, “I want my eyes to feel better,” and if dry eye is part of their diagnosis, we must actively manage their symptoms and try to address the patient’s chief complaint.
Dr. Kataria: Education on the importance of treating DED symptoms is crucial for both practitioners and patients. If patients present for a comprehensive examination and are given a sample of artificial tears and then sent home with no follow-up scheduled, then how can they comprehend how serious the condition is and what the ramifications may be for their vision?
Dr. Davidson: Patients behind the phoropter should not have to blink excessively to bring things into focus, nor should their eyes water. Those are cardinal signs of DED.
MANAGING DED
Dr. Quint: I’m curious as to how quickly you each initiate DED treatment in patients whom you newly diagnose. Is your initial visit purely educational? If you’ve just started the diagnosis process and have asked them to return for further testing, do you prescribe them a treatment in the interim, even if it’s over the counter?
Dr. Kataria’s Steps for Managing and Treating DED
1. Education. Inform the patient that you have diagnosed him/her with DED, using visuals such as corneal staining.
A. Describe the serious nature of DED and explain that it requires lifelong management.
B. Discuss at-home treatments, such as lid hygiene, computer hygiene, dietary changes, humidifiers, etc.
2. Follow-up. Have the patient return in 2-3 weeks for full testing, including meibography.
A. Determine the patient’s chief complaints and treatment goals.
B. Introduce the option of in-office treatments (IPL, etc.).
C. Establish the customized treatment plan.
3. Second Follow-up. Have the patient return in another 4-6 weeks to evaluate their progress.
A. What’s working? What isn’t? Is compliance to the protocol realistic for the patient?
B. How much have signs and symptoms improved?
C. Consider a prescription topical therapy.
Dr. Dierker: I don’t believe we can have a standardized starting point for every DED patient, because their needs differ, as do their levels of motivation for adhering to treatment. What is the patient’s chief complaint? What is the treatment goal? There are so many variables: does the patient have comorbidities that may be contributing to the DED? Are they preparing for an upcoming surgery? Are they trying to get back into contact lenses?
I try to create a customized treatment plan for each patient that they’re likely to follow, because adherence is everything. I think the best approach to DED treatment management is to determine the patient’s capacity for treatment compliance, and then to choose therapies targeted to treat the underlying disease process and address their chief complaints.
Dr. Kataria: I agree that our approach depends on the patient, and education is the first step (see the sidebar for Dr. Kataria’s steps for Managing and Treating DED). Patients must understand that DED can be serious, that it won’t resolve on its own, and that they will have to manage it chronically.
My first step in the DED diagnosis process is to give the patient their preliminary diagnosis and show them the pictures of their corneal staining to give them a visual of what’s happening on their ocular surface. I’ll suggest things they can do at home, like lid hygiene, computer hygiene, and dietary modifications. Then, I ask the patient to schedule a short follow-up visit. I’m a huge proponent of this short follow-up—it’s a great practice-builder, and it reinforces the message that their condition is serious. I have them return in 2 to 3 weeks to undergo the full battery of dry eye testing, including meibography, etc. My goals with the second visit are to determine the patient’s primary symptom that bothers them the most, as well as their treatment goals, and to establish a customized treatment plan. If needed, I introduce the option of in-office treatments for the meibomian glands, such as intense pulsed light (IPL) therapy and thermal evacuation. If those types of treatment are not an option for the patient, I still don’t start prescription therapeutics at that time. I give them an opportunity to comply with aforementioned treatments and schedule another follow-up to consider prescription eye drops. This protocol, is of course, tailored to each patient. I always go back to the TFOS DEWS II treatment algorithm.2
Dr. Quint: One of my best treatment strategies is to document what the patient’s goals are for fixing their DED. Do they want to be able to drive? To read? This is my starting point of treatment. Is anyone else implementing the DEWS II guidelines into their practice?
Dr. Schweitzer: I use the DEWS II recommendations as a guideline. They are crucial to have, because they establish effective protocols that any practitioner can follow. Yet, every patient needs slightly different interventions, because DED is a very complex disease.
I use most of my time with patients to educate them about DED, because I think it reinforces treatment compliance. Our treatment plans need to include contingency strategies if what we initially prescribe doesn’t work, and letting patients know we have a backup plan builds trust. If a prescription isn’t working, I usually bring my patients back in 4 to 6 weeks to take the next steps. Those steps are already established in the chart, and the patient is already educated on what they will be.
Dr. Dierker: We know that MGD is the leading cause of evaporative DED.4 I look at each component of the patient’s disease and try to address it, starting with inflammation. I evaluate whether the patient needs an immunomodulator, a topical steroid, or an amniotic membrane. Could they benefit from an in-office light-based treatment? Is there evidence of blepharitis on the lids or biofilm? Are the meibomian glands obstructed? What is the level of tear film instability? These are the main things I look for, and then I seek the appropriate targeted therapies for each issue.
Dr. Davidson: If I’m having trouble differentiating whether a patient has ocular allergies or some type of DED, I’ll have them sample an antihistamine drop in the office and wait 10 minutes. If their eyes feel even 10% better, then I know there’s an allergy component there.
NUTRITIONAL SUPPLEMENTS AND BLINK™ NUTRITEARS®
Dr. Quint: Many of our patients want to know about lifestyle actions they can take at home to help ease their symptoms of ocular dryness through nutrition, sleep, screen hygiene, gut health, etc. Recent studies have shown that an anti-inflammatory diet that includes particular nutrients can help mitigate some symptoms of ocular discomfort.5,6 Bausch + Lomb recently introduced Blink™ NutriTears® (Figure). Have any of you been recommending this supplement, or any nutritional supplement, in your practices?
Dr. Schweitzer: I agree we should discuss nutrition with patients, because more and more of them ask about it. I believe in using nutraceuticals as an adjunct to dry eye therapies.
Dr. Kataria: My patients are always asking about dietary modifications—it’s something that’s in their control. I have made nutrition part of my initial conversation with patients who show symptoms of dryness, and it plants the seed that these symptoms require more of a holistic and often systemic treatment approach.
Dr. Dierker: When I recommend BLINK NutriTears, I let patients know it’s to help support their tears from the inside out.* Patients like an affordable once-daily supplement that helps their symptoms. I don’t recommend it for every patient, but it is an important part of a management plan for many of my patients.

Figure. Blink™ NutriTears® nutritional supplement is formulated with a proprietary blend of key ingredients to help support the production of healthy tears.*,§
Dr. Davidson: I’ve done a deep dive into nutraceuticals over the past couple of years, and there’s definitely something to them. When the body is properly hydrated, the patient feels better systemically, and their eyes tend to feel better. I, too, like that Blink NutriTears is a once-a-day formula. There are many great supplements out there, but taking 3 to 4 pills a day is a challenge for anyone. I agree with Dr. Dierker, again, that using nutraceuticals to alleviate symptoms of ocular dryness from the inside out is very powerful.
Dr. Quint: Every patient of mine who uses Blink NutriTears has commented, “It is nice for once a day, and it’s an easy pill to take.”
*These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease.
§Based on a clinical study.
MIEBO® CLINICAL EXPERIENCE
Dr. Quint: Next, we’ll discuss MIEBO® (perfluorohexyloctane ophthalmic solution) (Bausch + Lomb) and XIIDRA® (lifitegrast ophthalmic solution) 5% (Bausch + Lomb), and how we use these two treatments in our practices.
Let’s start with MIEBO, which is the first and only prescription eye drop for the treatment of the signs and symptoms of DED to directly target evaporation.7 MIEBO is available in QID dosing. Perfluorohexyloctane is a unique molecule that provides evaporative relief. Preclinical pharmacokinetic studies have shown it can stay on the surface of the eye for at least 6 hours.4 In my practice, I find MIEBO is very well tolerated, which is important for dry eye patients.
When are you using MIEBO? Has it become your first-line therapy? And, what is your typical patient profile for MIEBO?
Dr. Davidson: Although there is no first-line therapy that works for all dry eye patients, I prescribe MIEBO frequently for post-LASIK patients, for whom I’ve found it works very well. I even used MIEBO myself after I received the Visian ICL (STAAR Surgical).
Dr. Dierker: When considering any prescription medication, I think it’s useful to look at the type of patients who took part in the clinical trials. In the phase 3 GOBI and MOJAVE studies for MIEBO, the patients were adults who had a diagnosis of DED for at least 6 months, as well as an MGD score of ≥ 3 on a scale of 0-15, in addition to corneal fluorescein staining.9,10 This is representative of a majority of patients in my population. So, I consider MIEBO a first-line therapy for every patient who has failed on artificial tears and who has signs and symptoms of evaporative DED and MGD. MIEBO has a lot of appeal because of its broad treatment label and its tolerability profile,11 and I’ve seen it work pretty quickly in terms of improving symptoms (Figure 3). It can also have a robust action on corneal staining (Figure 4). I’ve seen older medications have issues with both access and tolerability, but I have not encountered either one with MIEBO.
Please see the Indication and Important Safety Information for MIEBO and XIIDRA at the end of this supplement
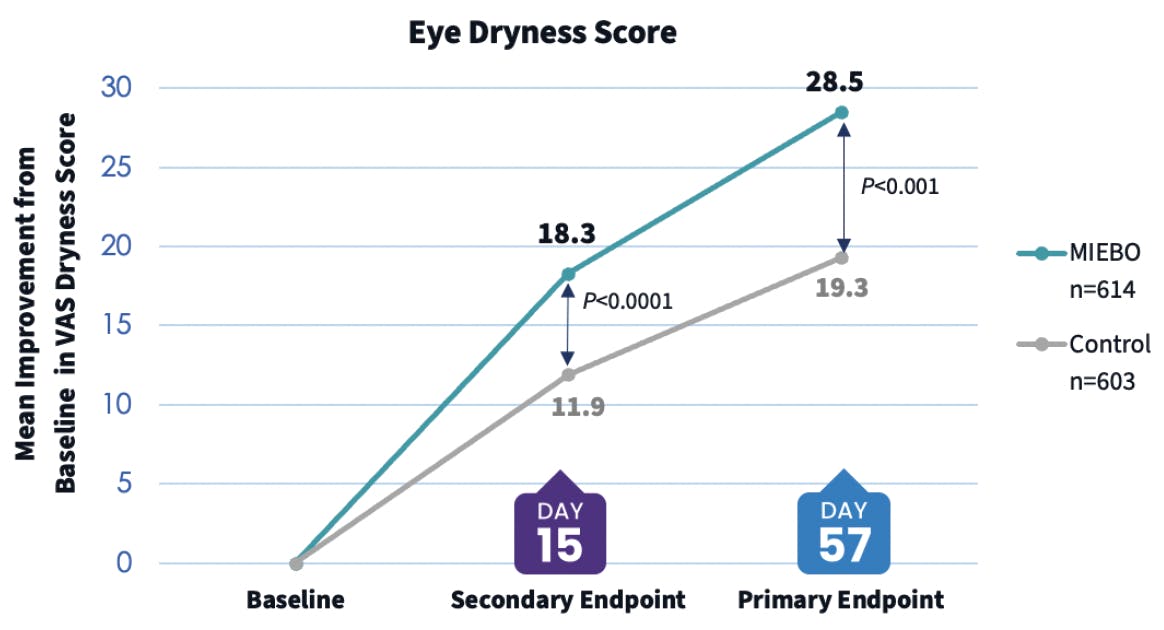
Figure 3. The pooled visual analog score (VAS) eye dryness data from the GOBI and MOJAVE phase 3 clinical trials showed that MIEBO provided rapid and sustained relief from ocular dryness through day 57, which was a primary endpoint. In a secondary endpoint, this relief was seen as early as day 15.9,10
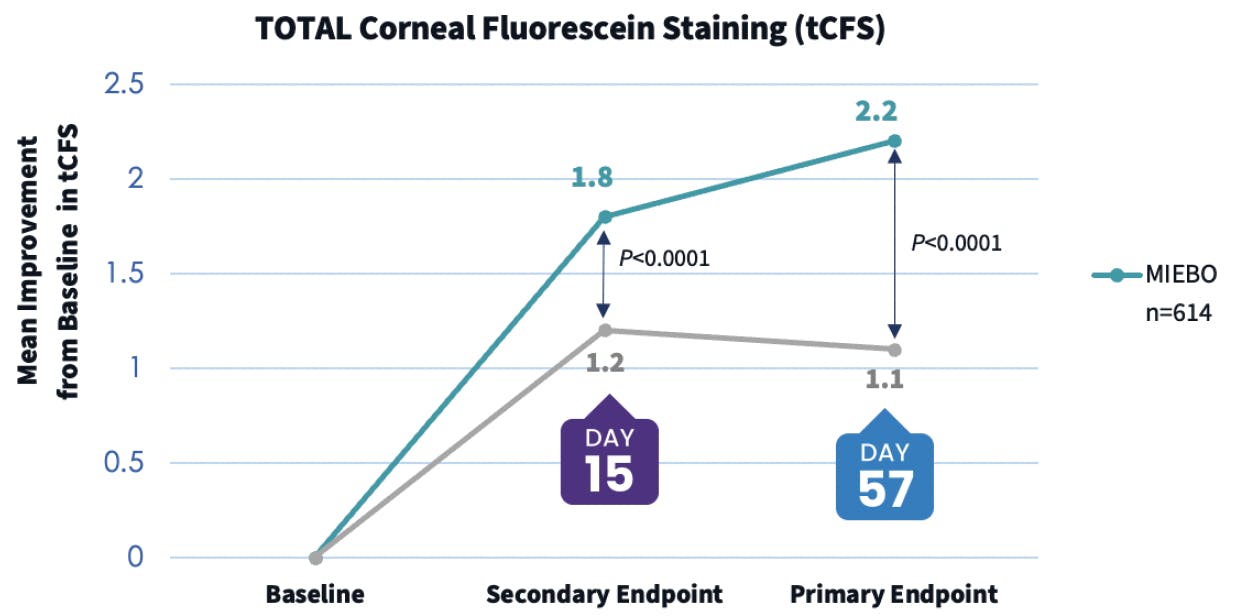
Figure 4. The pooled tCFS data from the GOBI and MOJAVE phase 3 clinical trials showed that MIEBO provided a sustained improvement in corneal staining through day 57, which was a primary endpoint. In a secondary endpoint, this improvement in corneal staining was seen as early as day 15.9,10
In my clinic, patients’ experience with MIEBO has been overwhelmingly positive. It may not treat every symptom for every patient, but it’s a nice first step for many of them. In a referral center or a tertiary care dry eye practice, I think MIEBO is a nice add-on for tough-to-treat patients, such as those with fluctuating vision later in the day.
Dr. Schweitzer: I agree. I prescribe MIEBO for DED patients who have associated MGD. First, I will treat these individuals with an in-office therapy, such as IPL, thermal pulsation, or a heat and gland-clearing system to treat the meibomian glands. I follow my in-office therapy with a MIEBO prescription. Dr. Dierker mentioned the corneal staining results from the MIEBO clinical trials; I’ve found it’s very important to improve corneal staining and have seen improvement in staining in my clinic with these patients.
Dr. Kataria: I completely agree—I also use MIEBO following in-office therapies for MGD. The feedback from these patients has been very positive: MIEBO makes their blinks feel smooth. And then, as Dr. Dierker described, MIEBO is great for treating the visual fluctuations patients may experience throughout the day, especially in those frictional dry eye conditions. So, for my clinic, MIEBO has various applications.
Dr. Quint: How do you set patients’ expectations for what their experience with MIEBO might be?
Dr. Davidson: MIEBO is one of those few drops that I’ll let patients sample in the office. I’ll ask them to blink a few times after instillation, and often, they’ll quickly notice a little improvement in their vision.
Dr. Dierker: I know that MIEBO is an anti-evaporative drop, but I explain to patients that it’s a tear stabilizer. In the clinical trials for MIEBO, the control was hypotonic saline, which has similar properties to artificial tears. Based on the pooled dataset, MIEBO outperformed the hypotonic saline at every time point in both signs and symptoms.12 As Dr. Quint mentioned, the preclinical data show that MIEBO stays on the ocular surface for at least 6 hours, so I don’t see the need to add an OTC tear product on top of it. I want to choose something that has a high rate of success and patient tolerability. I want the patient to be happy when I see them back again, and in my experience, most patients respond positively to MIEBO.
Dr. Schweitzer: Tolerability is a key factor to talk about with patients. In its clinical trials, the biggest side effect of MIEBO was blurry vision in 13 patients out of 614 (Figure 5). From a tolerability standpoint, MIEBO makes me feel very confident.
![<p>Figure 5. MIEBO showed an excellent tolerability profile in two pivotal clinical studies of more than 1200 patients (more than 600 of whom received MIEBO). (1. Tauber J, et al. <em>Ophthalmology</em>. 2023;130(5):516-524. 2. Sheppard JD, et al. <em>Am J Ophthalmol</em>. 2023;252:265-274.) (*Data were pooled from 2 pivotal clinical studies [GOBI and MOJAVE]. Each study had >600 patients treated with MIEBO. The analysis took into account >1200 total patients. Of the 614 patients who received MIEBO, there were no incidences of serious ocular adverse events (AEs) with MIEBO. Most AEs were considered mild. The discontinuation rate for MIEBO was comparable to control [pooled: 0.2% vs 0.5%; GOBI: 0.3% vs 1.0%; MOJAVE: 0% vs 0%]. Instillation site pain AEs, such as burning and stinging, were reported by 0.5% [pooled] patients [GOBI: 1.0%; MOJAVE: 0%]. Blurred vision [pooled: 2.1%; GOBI: 3.0%; MOJAVE: 1.3%] and conjunctival redness [pooled: 0.8%; GOBI: 0%; MOJAVE: 1.3%] were reported in 1%-3% of individuals.)</p>](https://cdn.reachmd.com/uploads/modernod/articles/Screenshot_2025-03-20_1742499444.png)
Figure 5. MIEBO showed an excellent tolerability profile in two pivotal clinical studies of more than 1200 patients (more than 600 of whom received MIEBO). (1. Tauber J, et al. Ophthalmology. 2023;130(5):516-524. 2. Sheppard JD, et al. Am J Ophthalmol. 2023;252:265-274.) (*Data were pooled from 2 pivotal clinical studies [GOBI and MOJAVE]. Each study had >600 patients treated with MIEBO. The analysis took into account >1200 total patients. Of the 614 patients who received MIEBO, there were no incidences of serious ocular adverse events (AEs) with MIEBO. Most AEs were considered mild. The discontinuation rate for MIEBO was comparable to control [pooled: 0.2% vs 0.5%; GOBI: 0.3% vs 1.0%; MOJAVE: 0% vs 0%]. Instillation site pain AEs, such as burning and stinging, were reported by 0.5% [pooled] patients [GOBI: 1.0%; MOJAVE: 0%]. Blurred vision [pooled: 2.1%; GOBI: 3.0%; MOJAVE: 1.3%] and conjunctival redness [pooled: 0.8%; GOBI: 0%; MOJAVE: 1.3%] were reported in 1%-3% of individuals.)
Dr. Davidson: Our patients find MIEBO tolerable, and it’s relatively easy for them to get. That’s a home run for our clinic.
Dr. Quint: I like that MIEBO doesn’t have any contraindications, and it can be used in conjunction with other drops such as glaucoma medications. That can be a really important point to bring up with patients.
Dr. Dierker: I’ve rarely had a patient who couldn’t tolerate MIEBO. Occasionally I’ve encountered a patient who experiences some blurry vision temporarily after instillation, but it’s a different patient experience than we’ve had with other prescription eye drops.
XIIDRA® CLINICAL EXPERIENCE
Dr. Quint: We often see inflammation as a component of DED—the disease is an inflammatory process.13 Some patients have a level of ocular inflammation that requires an immunomodulator.
XIIDRA® (lifitegrast ophthalmic solution) 5% (Baush + Lomb) is indicated for the treatment of signs and symptoms of dry eye disease (DED).14 It is an immunomodulator with a unique mechanism of action: it interferes with the activity of both active and inactive T cells to tamp down the inflammatory response. And, it is the only prescription medication that can inhibit T-cell adhesion to ICAM-1 to target inflammation in the eyes.*,14,15
For those of you who have used XIIDRA in your practices, what does your patient profile look like?
Dr. Schweitzer: My patient profile for XIIDRA is someone who has corneal staining, a positive MMP-9 test, abnormal osmolarity, and essentially normal meibomian glands.
Dr. Davidson: I have found XIIDRA to be effective when I have a patient who responds well to steroids, and then, upon removal of the steroid, other treatments just aren’t doing the trick.
Dr. Dierker: Like MIEBO, XIIDRA has a broad label for treating the signs and symptoms of DED. I think patients who have signs of ocular inflammation, including corneal and conjunctival staining, and particularly if they have elevated MMP-9 scores, are good candidates for XIIDRA. The more complex a patient’s disease, the more they have systemic comorbidities or even systemic inflammatory diseases, the more likely I am to prescribe XIIDRA. Also, it tends to be my go-to medication for lingering symptoms that persist despite in-office treatments and other therapies.
My classic patient profile for MIEBO as a first-line therapy tends to skew toward younger patients who are generally healthy, and maybe their dry eye is mostly due to increased evaporation secondary to digital device use. If, however, they have signs of inflammation on their clinical exam, I’m more likely to prescribe XIIDRA.
Dr. Kataria: Sometimes, it’s very appropriate for XIIDRA to be our first-line therapy. If I see corneal staining, I won’t hesitate to prescribe XIIDRA. Other times, I will implement other in-office therapies and use XIIDRA as adjunct therapy.
Dr. Dierker: Preoperative patients are important candidates for XIIDRA. With it, we can get more predictable corneal measurements for IOL planning, and relatively quickly. In addition to treating DED signs and symptoms, stabilizing and optimizing the ocular surface prior to surgery is a sweet spot for XIIDRA in my practice.
Dr. Quint: We have a lot of data on XIIDRA—it’s been used clinically since 2016, and it has been well studied. In two of four clinical trials, XIIDRA demonstrated relief from ocular dryness in as little as 2 weeks, and within 6 to 12 weeks in four clinical trials (Figure 6). Not every patient experiences such a rapid effect, but because XIIDRA has the potential to work so quickly, I tend to reach for it to improve the ocular surface of patients with cataracts before I refer them for surgery. Do the 2-week data on XIIDRA influence any of your prescribing habits?
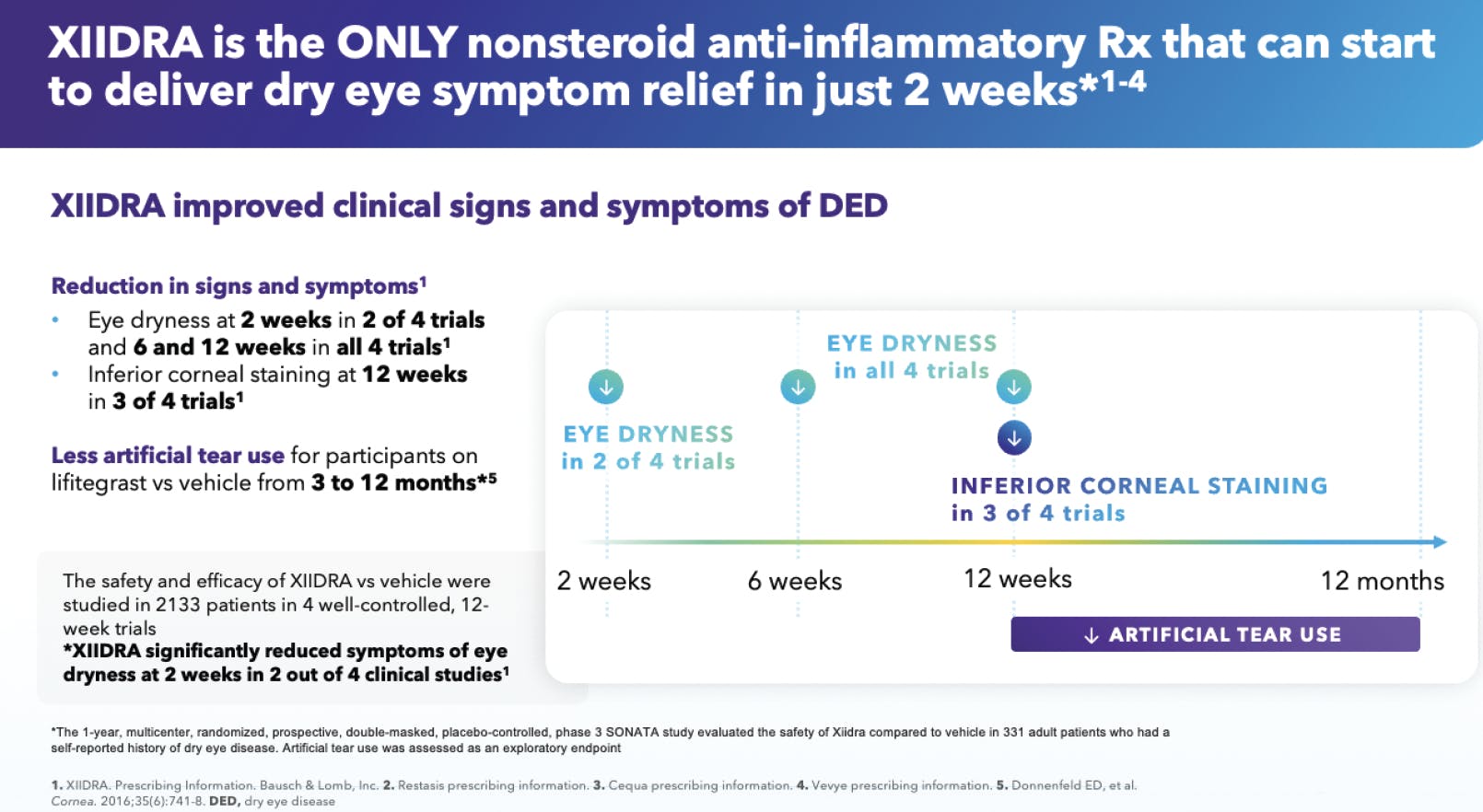
Figure 6. XIIDRA acts quickly. Four clinical trials assessed patients’ improvement in signs (measured by inferior corneal staining score) and symptoms (measured by eye dryness score) of DED (n=2,133). In two of those trials, XIIDRA improved symptoms of DED in as little as 2 weeks. (1. XIIDRA prescribing information).
Dr. Schweitzer: These data matter and do influence my prescribing patterns for the presurgical patient. Surgical candidates don’t want to wait an extended period of time to have cataract surgery and see better. Any treatment that can improve the corneal surface in a short amount of time is an advantage.
Dr. Davidson: In any type of patient, the faster we achieve relief, the better. That’s why XIIDRA’s 2-week data really spoke to me.
Dr. Kataria: The efficacy of XIIDRA is excellent. I find that, if a patient on XIIDRA is not experiencing some relief within 2 weeks, it’s often because they have other comorbidities that are contributing to their symptoms.
Dr. Quint: Clinically, how do you find XIIDRA’s tolerability profile? Do you need to set any patient expectations with XIIDRA?
Dr. Dierker: We have to let the patient know what we’re trying to achieve with any medication we prescribe, and what potential side effects may be. The more patients anticipate adverse reactions with a medication, the better they tolerate them. For XIIDRA, I inform them that they may feel some burning/stinging when they first start using it, and they may have an abnormal taste sensation or blurry vision temporarily. Those phenomena tend to be mild and get better with time. Proactive education makes it less likely that the symptoms will be a problem for that patient. I have even encountered patients who have these symptoms, such as dysgeusia, but the relief they feel from XIIDRA outweighs the negative.
Dr. Kataria: I agree, and I would add that having the staff involved in setting patients’ expectations at the initial prescription visit is crucial. The more we reinforce this expectation, the less of a surprise symptoms are to patients.
TREATMENT SELECTION FOR DED PATIENTS
Dr. Quint: We’ve talked about how MIEBO and XIIDRA have different mechanisms of action. How do you approach the patient who needs both treatments? What are your strategies?
Dr. Dierker: This is a common clinical scenario in my practice, where I treat patients with more moderate-to-severe disease. When I see inflammation as the most critical factor in a patient’s symptoms, I tend to reach for XIIDRA first, and then I’ll address their other symptoms, including tear evaporation if that persists. Many times, these individuals don’t need anything beyond XIIDRA. As I described earlier, I use MIEBO first in patients who are younger, have not been on prescription medications before, and show very little inflammation.
Dr. Kataria: I agree with Dr. Dierker’s protocol, with the caveat that I’ll stop the XIIDRA if the patient reacts to it. If I deem that a patient would benefit from both therapies, but that person is highly anxious and/or sensitive to the side effect profile, then I’ll start with MIEBO first.
Dr. Dierker: Dr. Kataria’s point is critical: we’re treating a patient, not just the corneal staining or the MGD. With people, there are a lot of factors to consider. It’s a boon to us eye care practitioners to have these targeted therapies to choose from.
Dr. Schweitzer: Compliance is an issue for many patients, so I avoid overwhelming them with drops. If I see mild corneal staining and signs of evaporative DED, I lean toward starting the patient on MIEBO, because of its clinical data showing that it improves corneal staining and directly prevents tear evaporation. If I see staining (indicating the presence of inflammation) without evaporative DED, I lean toward prescribing XIIDRA.
Dr. Quint: My approach is always to put out the bigger fire first, whether that’s inflammation or evaporation. We can always add other treatments as needed. And, I agree that we have to be realistic about the compliance our patients are capable of.
Dr. Quint: How do MIEBO and XIIDRA fit with in-office treatments, such as thermal pulsation, light therapies, etc?
Dr. Dierker: We know that MIEBO and XIIDRA work well in isolation. Still, they may work best when we can identify and address the root causes of the patient’s ocular surface disease. I use MIEBO and XIIDRA as primary therapies, but I also will use them routinely as adjunctive therapies to my root-cause treatment—a nice benefit of their broad indication labels.
What Do You Wish Patients Knew About DED?
What messages should we be sharing with our patients about DED?
Dr. Davidson: There is no silver-bullet cure for DED, no single treatment that will fix all DED symptoms for all patients. At the same time, patients don’t have to live with chronic ocular discomfort. They shouldn’t be thinking about how their eyes feel every day. We have the ability now to treat these symptoms.
Dr. Quint: I would say to patients, if you have any ocular symptoms, talk to an eye care professional. Let somebody knowledgeable guide you through your treatment options, because the knowledge base on managing DED is growing all the time.
Dr. Dierker: I know that many patients are frustrated because they’ve tried to have these conversations with their eye care providers in the past, and they weren’t very fruitful. I wish they would try again, because we have better doctor education, and we have more treatment options for mild, moderate, and severe disease. We want to help them, and we’ve got better tools to do it.
Dr. Kataria: There is a large psychosocial burden of understanding and treating DED from the patient’s perspective. We have many tools that can help providers educate our patients better and reduce this burden by offering appropriate therapies.
CONCLUSION
Dr. Quint: What do we wish our optometric colleagues knew about DED or these treatments that we’ve discussed?
Dr. Dierker: With the tools that we have at our disposal, practitioners should not hesitate to diagnose and treat DED.
Dr. Davidson: Dry eye can be a differentiator for your clinic.
Dr. Quint: Don’t be afraid to use adjunct therapies. Be able to pivot if it’s not working.
Dr. Schweitzer: We have great prescription treatment options; we no longer need to rely on artificial tears for every patient.
Dr. Quint: One thing I like about the outcomes we’ve had with prescription options is that providers can still address the patient’s symptoms without having specialty dry eye treatment equipment. Every eye care provider already has a slit lamp. The slit lamp plus a good protocol for identifying DED patients is a good start to addressing a patient’s dry eye needs.
Dr. Kataria: I would love to highlight to my colleagues that DED is a chronic condition and must be managed as such. Follow it appropriately and bring those patients back for follow-up.
Dr. Dierker: DED is common and can have a negative impact on a patient’s quality of life. So, I think we should be spending appropriate time and energy to identify and treat these patients.
1. Harris poll: Dry Eye Research Report. unpublished data on file with Bausch + Lomb, 2024.
2. TFOS DEWS II Patient Summary. Tear Film and Ocular Surface Society. July 4, 2019. Accessed October 29, 2024. https://www.tearfilm.org/dettnews-tfos_dews_ii_patient_summary/6814_5519/eng/.
3. Guo LW, Akpek EK. The negative effects of dry eye disease on quality of life and visual function. Turk J Med Sci. 2020;50(7):1611–1615.
4. Sheppard JD, Nichols K. Dry eye disease associated with meibomian gland dysfunction: focus on tear film characteristics and the therapeutic landscape. Ophthalmol Ther. 2023;12:1397–1418.
5. Cong Y, Zhang Y, Han Y, et al. Recommendations for nutritional supplements for dry eye disease: current advances. Front Pharmacol. 2024;15:1-17.
6. Rand AL, Asbell P. Nutritional supplements for dry eye syndrome. Curr Opin Ophthalmol. 2011;22(4):279-282.
7. MIEBO. Prescribing Information. Bausch + Lomb.
8. Kroesser S, Spencer E, Grillenberger R, et al. Ocular and systemic distribution of 14c- perfluorohexyloctane following topical ocular administration to rabbits ARVO Annual Meeting Abstract. Invest Ophth Vis Sci. 2018;59(9):2656.
9. Tauber J, Berdy GJ, Wirta DL, et al. NOV03 for dry eye disease associated with meibomian gland dysfunction: Results of the randomized phase 3 GOBI study. Ophthalmology. 2023;130(5):516-524.
10. Sheppard J, Kurata F, Epitropoulos AT, et al. NOV03 for signs and symptoms of dry eye disease associated with meibomian gland dysfunction: The randomized phase 3 Mojave study. Am J Ophthalmology. 2023;252:265-274.
11. Bausch + Lomb data on file: DOF/Memo supporting data for NOV03 spreading and comfort on instillation/p2.
12. Fahmy AM, Harthan JS, Evans DG, Greiner JV, Tauber J, Sheppard JD, Krösser S, Vittitow JL. Perfluorohexyloctane ophthalmic solution for dry eye disease: pooled analysis of two phase 3 clinical trials. Front Ophthalmol (Lausanne). 2024 Nov 5;4:1452422. .
13. Wei Y, Asbell PA. The core mechanism of dry eye disease (DED) is inflammation. Eye Contact Lens. 2014;40(4):248–256.
14. XIIDRA. Prescribing information. Bausch + Lomb.
15. Perez VL, Pflugfelder SC, Zhang S, et al. Lifitegrast, a novel integrin antagonist for treatment of dry eye disease. Ocul Surf. 2016;14(2):207-215.
NUTRITEARS is a trademark of OmniActive Health Technologies Ltd., used under license.
Xiidra® (lifitegrast ophthalmic solution) 5%
INDICATION
Xiidra® is indicated for the treatment of signs and symptoms of dry eye disease (DED).
IMPORTANT SAFETY INFORMATION
• Xiidra is contraindicated in patients with known hypersensitivity to lifitegrast or to any of the other ingredients.
• In clinical trials, the most common adverse reactions reported in 5-25% of patients were instillation site irritation, dysgeusia and reduced visual acuity. Other adverse reactions reported in 1% to 5% of the patients were blurred vision, conjunctival hyperemia, eye irritation, headache, increased lacrimation, eye discharge, eye discomfort, eye pruritus and sinusitis.
• To avoid the potential for eye injury or contamination of the solution, patients should not touch the tip of the single-use container to their eye or to any surface.
• Contact lenses should be removed prior to the administration of Xiidra and may be reinserted 15 minutes following administration.
• Safety and efficacy in pediatric patients below the age of 17 years have not been established.
Click here for full Prescribing Information for Xiidra.
MIEBO® (perfluorohexyloctane ophthalmic solution) INDICATION
MIEBO® is indicated for the treatment of the signs and symptoms of dry eye disease.
IMPORTANT SAFETY INFORMATION
• MIEBO should not be administered while wearing contact lenses. Contact lenses should be removed before use and for at least 30 minutes after administration of MIEBO
• Instruct patients to instill one drop of MIEBO into each eye four times daily
• The safety and efficacy in pediatric patients below the age of 18 have not been established
• most common ocular adverse reaction was blurred vision (1% to 3% of patients reported blurred vision and conjunctival redness)
Click here for full Prescribing Information for MIEBO.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
© 2025 Bausch + Lomb MBO.0100.USA.25




_1738693553.png)


