INTRODUCTION
Selina R. McGee, OD, FAAO: Dry eye disease (DED) commonly arises from excessive tear evaporation exacerbated by meibomian gland dysfunction (MGD).1-6 The resultant instability of the tear film leads to an increase in ocular surface desiccation and inflammation.
In May, the FDA approved MIEBO (perfluorohexyloctane ophthalmic solution, Bausch + Lomb), the first and only prescription DED treatment that directly targets tear evaporation. MIEBO is different from existing prescription approaches, including immunomodulators, tear stimulators, and antiinflammatory agents. It is a single-ingredient, water-free, preservative-free, steroid-free formulation consisting of 100% active ingredient that is designed to curtail tear evaporation at the ocular surface and mitigate the adverse effects of evaporative DED.
MIEBO represents a stride forward in addressing an unmet need for millions of individuals suffering with the pervasive condition of DED. Its availability is an important advance in DED management. All the panelists here have experience prescribing MIEBO, and our discussion will illustrate the need for a targeted evaporative DED treatment such as MIEBO, review clinical data, and suggest routine protocols in targeted patient profiles.
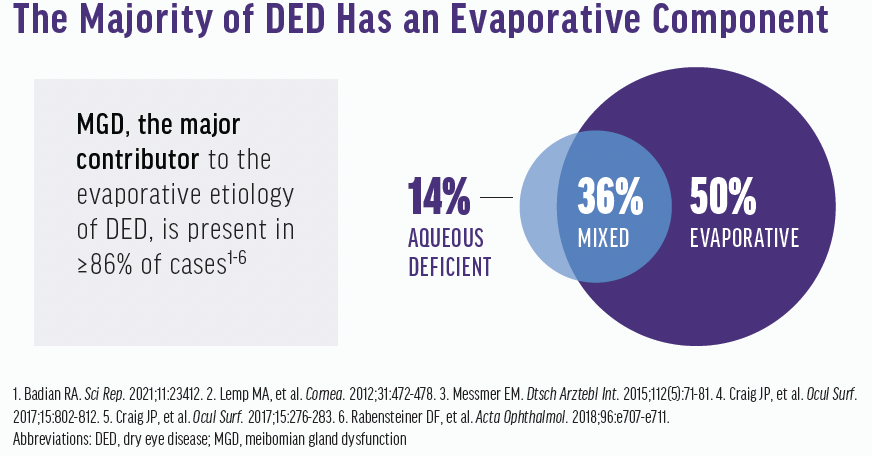
According to Lemp et al, 86% of DED patients have an evaporative component, whereas 14% have aqueous deficient DED (Figure 1). About 50% have pure evaporative DED, and 36% have mixed (ie, aqueous and evaporative).7
Following the advice of Dr. Hom, I approach every patient with the mindset that they have DED until proven otherwise. Milton, walk us through why you practice this way.
Milton M. Hom, OD, FAAO: For years, middle-aged and older women have been typecast as the most likely to have DED. This is because many DED studies were conducted no later than 2007, before smartphones were available.8 The real patient base, however, goes far beyond the older woman. We should be looking for DED in every patient, including baby boomers, millennials, and children. One reason so many individuals suffer from DED and MGD is the use of digital devices.
IDENTIFYING PATIENTS
Dr. McGee: It’s important not to typecast patients. I’m curious, how do you uncover patients who have DED, Melissa?
Melissa Barnett, OD, FAAO, FSLS, FBCLA: I find that a questionnaire is helpful to rule out dry eye or diagnose DED in every single patient. Individuals should repeat the same questionnaire in 1 month to 3 months to determine if their symptoms have improved.
Dr. McGee: Jim, when you’re behind the slit lamp, do you assess the meibomian glands and ocular surface in every single patient?
James Thimons, OD: My patients typically fall into two categories. Either they are elderly and almost universally have glaucoma, or they are referred to me because they’ve been on other DED therapies that have been unsuccessful. I find a thorough analysis of the posterior lid with the slit lamp is incredibly revealing. I believe that addressing the disease one patient at a time with a slit-lamp examination and gland assessment is the most effective way to identify the problem.
Dr. McGee: Derek, what do you think about when you hear multifactorial, and how do you approach the disease state?
Derek N. Cunningham, OD, FAAO: A constellation of etiologies are in play with DED, leading to a nebulous definition. Regardless of why the tissue is under stress for extended periods of time (eg, placement of eyeliner over the meibomian glands, frictional stress from tear deficiency, desiccative stress from environmental factors), the repeated effect of stress cascades to inflammation. We can calm the inflammation, but we cannot stop the root cause of the inflammation.
When I walk in the exam room, I pay as much attention to patient history and their skin type as to what I see at the slit lamp, because DED is heavily dependent on skin type. We know meibomian glands fit into the genesis of dry eye, and these glands are subtle derivatives of the sebaceous glands controlled by the same hormones.
I also like to determine if patients have any systemic disease, connective tissue disorders, rheumatological disease, or are immunocompromised, as well as what kind of anti-inflammatory capabilities the patient has. All these things play into their susceptibility to DED, as well as their potential success rate with conventional therapies.
Dr. Hom: To me, multifactorial means multi-confusion. We used to think that all DED was aqueous deficient. Now, however, we know that there are different factors, including lifestyle and inflammation. It gets even more confusing when you consider treatments.
Dr. McGee: We can’t forget that there’s a whole person behind the eyeballs. What they’re doing in their everyday life can affect their ocular surface. Many times, my patients have multiple coded diagnoses in their chart. It simplifies my treatment plan because I target the root cause of the patient’s symptoms. Instead of hoping for the best, now I customize their treatment plan.
Dr. Barnett: The more we look for specific components of ocular surface disease, the more we code for many different conditions at the same time. A more individualized approach is what we need to do to accurately treat DED.
CHALLENGES WITH TRADITIONAL DED THERAPY
Dr. McGee: For patients with aqueous deficiency, tear evaporation exceeds available supply, which disrupts the tear film integrity. The most common thing these patients complain of is fluctuating blurry vision, but they don’t correlate it with what’s happening on their ocular surface. They don’t even know what dry eye is typically. It’s important to educate patients in a way that compels them to understand that their vision is related to the front surface of the eye. Whether it’s aqueous deficient or evaporative DED, at the end of the day the patient wants to see clearly. If they don’t have a good quality tear film and it evaporates too quickly, then there’s a problem.
Dr. Barnett: I talk to my patients about the homeostasis of the tear film because it’s a fundamental component of DED. I get them to understand the term homeostasis or balance and that the tear film is the first refracting surface. I tell them that they need a stable tear film to maintain ocular surface health and good vision because it delivers nutrients, protects and moisturizes the ocular surface, and provides a smooth surface for the refraction of incoming light. It also prevents infection.
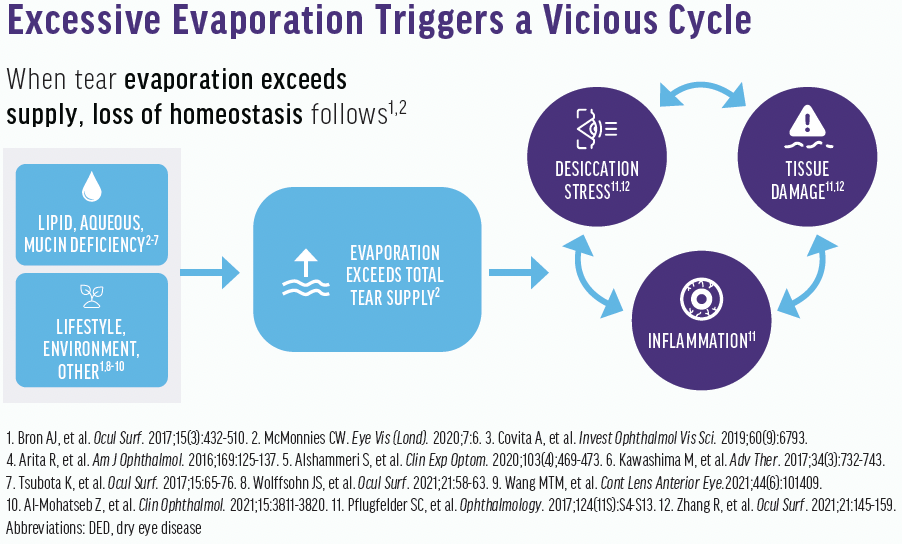
The tear film is made up of lipids, proteins, and electrolytes. In the presence of MGD, an alteration of the outer lipid-containing layer results in tear film instability. Additionally, excessive tear evaporation from lipid deficiency in MGD adversely affects the tear film lipid layer (Figure 2). I want patients to understand why we want to maintain integrity of the tear film and provide tear film homeostasis.
Dr. McGee: There is a huge gap in the number of individuals living with DED in the United States (38 million) and the number of those who are diagnosed and treated (18 million and 1.2 million, respectively).9-11
Historically with prescription therapies, immunomodulators and tear stimulators are used to target the inflammatory process and promote the production of natural tears, which are crucial for the integrity of the tear film, respectively. Anti-inflammatory agents can be used as well, but until recently there has never been a prescription drop therapy that directly targets the evaporative component of DED.
Dr. Thimons: The quality of the eyelid tissue is critical. Without a good lid ocular surface interface, the distribution of the tears and the lipid component can be disturbed independent of the quality of the product. For many of my patients with evaporative disease, the etiology is a combination of poor tear quality and poor maintenance of the ocular surface. Patients with DED and glaucoma are particularly challenging to treat. Their therapy frequently induces inflammation, further exacerbating their ocular surface disease issues. For this reason, selective laser trabeculoplasty (SLT) has become a first-line therapy.
MIEBO FOR GLAUCOMA PATIENTS
JIM THIMONS, OD
Historically, patients on multiple glaucoma medications with progressive ocular surface discomfort and chronic symptomatology are prescribed cyclosporine. Obviously, steroids are not a great choice for this group because of the complications of chronic use. I really love the potential of MIEBO to improve the ocular surface for our patients.
If I can help to restore ocular surface homeostasis, my hope is that their compliance with glaucoma therapy will improve. Right now, the biggest cause of glaucoma progression is lack of compliance, and the biggest cause of lack of compliance is that drops are irritating.
For glaucoma practitioners who see a lot of patients with DED, this drug can be a huge step forward.
Dr. McGee: To your point, it’s not just patients with dermatochalasis, poor lid seal, and decreased blink reflex who are looking for lasting relief, but it’s also patients who use neurotoxin in the orbicularis oculi.
Dr. Hom: Meibum can’t be dispensed into the ocular surface unless the patient blinks. So, if you don’t blink, you won’t get any meibum and you’ll get evaporative DED. It’s as simple as that.
Dr. Barnett: In today's digital age, we are not blinking a normal blink, which reduces tear film stability. In one study, 97% of computer users had poor stability of the tear film.12
MIEBO’S MECHANISM OF ACTION
Dr. McGee: MIEBO is the first FDA-approved prescription drop that directly targets tear evaporation. Melissa, why are you excited about MIEBO?
Dr. Barnett: MIEBO works to treat DED by preventing excessive tear evaporation. It stabilizes the tear film on the ocular surface so that tear evaporation is inhibited to prevent the eyes from drying out. MIEBO consists of 100% perfluorohexyloctane, which is a molecule with two different segments, one of which is lipophilic and one of which is aerophilic. The lipophilic portion embeds into and interacts with the lipid layer of the tear film while the aerophilic portion points out into the air. I'm excited because it's the first approved prescription drop for dry eye disease that works by reducing excessive tear evaporation caused by meibomian gland dysfunction.
Dr. Cunningham: MIEBO provides a shielding effect to the evaporative stress of dry eye. This is something we’ve really never had before. Sure, artificial tears provide a barrier, but it’s generally on the order of 3 to 5 minutes. Additionally, excessive artificial tear use washes out many pro-growth hormones in the eye. Not only do I think MIEBO is an attractive standalone therapy in some cases, but I think it has potential to be used with other DED therapies.
Dr. McGee: It’s a very interesting molecule. It’s 100% perfluorohexyloctane and preservative-free. There’s no pH, no inactive ingredients, no steroids, no water. It’s an interesting way that we haven't had before to fill a gap.
SAFETY, EFFICACY, AND TOLERABILITY
Dr. McGee: In two pivotal phase 3 clinical trials (GOBI and MOJAVE) involving more than 1,200 patients with DED and clinical signs of MGD, MIEBO consistently demonstrated safety and efficacy in addressing the clinical signs and patient-reported symptoms of DED.13,14 All patients had to provide a 6-month self-reported history of DED and have a total MGD score greater than 3 on a 0-15 scale. Patients with active blepharitis, those who wore contact lenses, those with a recent history of punctal plugs or MGD procedure, and those on any topical steroid, serum tear, other dry eye drug, or glaucoma medication were excluded from the study.
Patients were randomized one-to-one to receive MIEBO or a control four times a day. The primary endpoints were changes from baseline in total corneal fluorescein staining (tCFS) and improvements in visual analog scale eye dryness. Both studies met their primary endpoints demonstrating that MIEBO improved the signs and symptoms of DED. A rapid and sustained improvement in both tCFS and eye dryness was reported as early as day 15 through day 57 (Figure 3). There were no serious adverse events and a 0.2% rate of discontinuation due to ocular adverse events, which is extremely low. About 0.5% of patients reported an adverse event of burning or stinging on drop instillation. The most common ocular adverse events were blurred vision, mostly mild and transient, and eye redness, which were reported in 1-3% of patients.
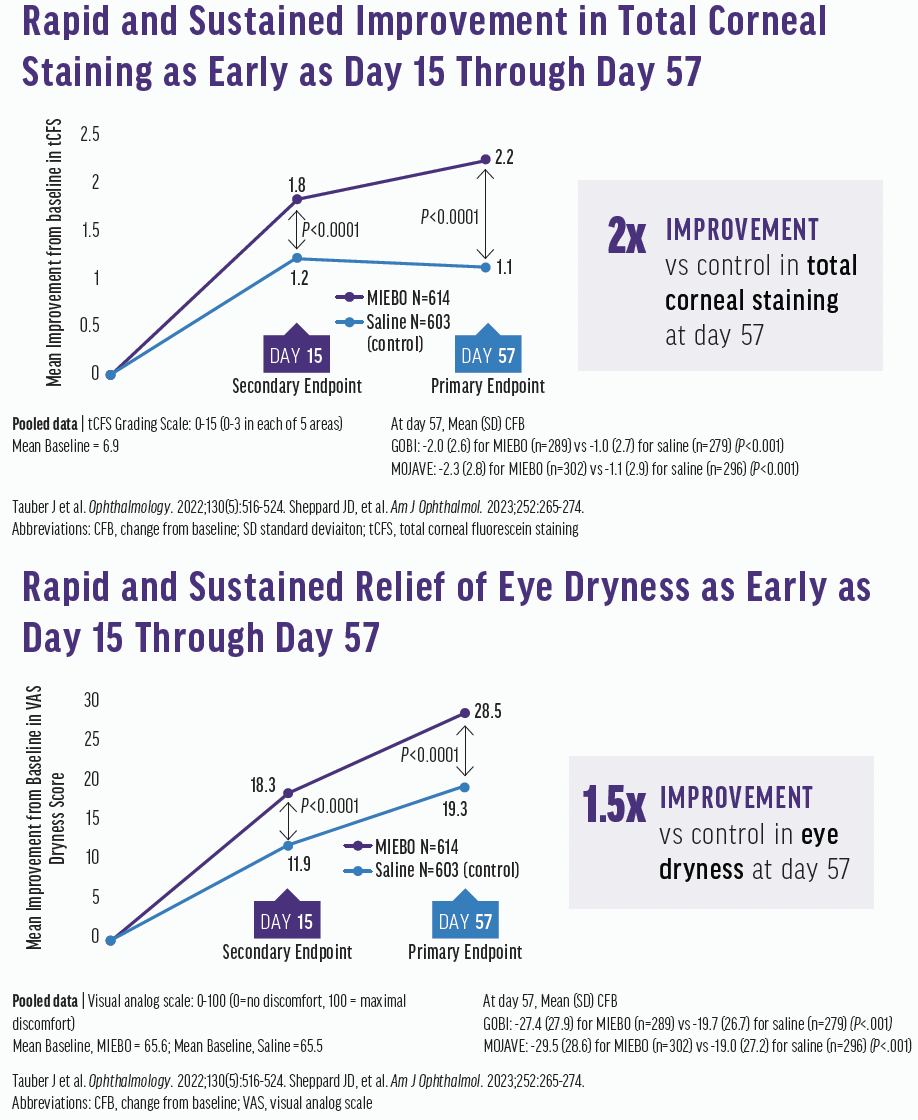
Figure 3. Improvements in total corneal fluorescein staining (top) and eye dryness (bottom) scores were seen at days 15 and 57.
Dr. McGee: Milton, how do you translate the study results to clinical practice?
Dr. Hom: When you look at the structure of the clinical study, there are two points: the sign and the symptom. The sign is corneal fluorescein staining, which shows improvement. The symptom is the eye dryness score. With MIEBO, the eye dryness score improved over time, as did corneal staining. So, MIEBO is really a dry eye medication.
Dr. Barnett: I’m excited that there were no serious ocular adverse events, and the discontinuation rate due to adverse events was extremely low. Additionally, instillation was very comfortable. Patients reported a low rate of burning or stinging.
Dr. McGee: Derek, what does this kind of data mean to you?
Dr. Cunningham: It’s a big deal. It confirms that we can help to normalize tissue quickly without doing anything that is going to disrupt the natural tear flow. It’s an interesting concept; we can allow the inflammatory factors to wash out of the eye while still providing desiccative stress relief.
Something that works in as quickly as 2 weeks, such as MIEBO, is an attractive option.
My entire staff has DED and started using perfluorohexyloctane. I think the wow factor is the real utility of what this drug can do. It’s impressive at the slit lamp—there’s no doubt about that—but it’s also going to give people longitudinal relief.
Dr. Barnett: It’s rare to see improvement at day 15 with immunomodulators. In my experience, it often takes 6 weeks to 3 months to see some improvement.
MIEBO TO OPTIMIZE THE OCULAR SURFACE BEFORE CATARACT SURGERY
DEREK CUNNINGHAM, OD
Before I walk in to evaluate any patient for cataract surgery, I look at their topography, which is like an A1C for DED. Topography can show how dry the eye has been for several weeks before they’ve come in because of the dehydration that happens regionally on the cornea. Additionally, a topography with incomplete data signals to me immediately that the patient has DED.
A dry cornea is shriveled up like a raisin instead of a grape, which leads to high calculation error margins. I put about 80% of my cataract surgery patients on an aggressive steroid before surgery. One issue with doing this, however, is it doesn’t allow enough time for the tissue to hydrate.
Additionally, I use a punctal plug early in the treatment phase if the patient has other factors besides inflammation. MIEBO is an interesting addition to help maintain natural tear homeostasis by providing a shield effect to the eye. It can greatly increase our surgical results because a better ocular surface improves the accuracy of our calculations.
Ultimately, I don’t take patients off MIEBO after cataract surgery if their cornea responded positively to treatment effect. This is a medication that has low systemic absorption, it has an excellent safety profile, and we don’t want the patient’s cornea to go back to its preoperative status. To me, MIEBO is not only a pretreatment drug for a lot of my cataract patients, this is certainly one that I keep them on long-term afterward.
Dr. Thimons: We have a large referral network, and the patients we treat frequently have ocular surface-related issues. When the ocular surface is irregular, we are unable to capture perfect preoperative measurements before cataract surgery. The variability between an eye with untreated DED can even be up to 1.00 D at 3 months.15 Most patients won’t wait 3 months to have surgery. MIEBO is a great opportunity for primary eye care physicians to prep the cornea for cataract surgery in patients with dry eye disease. The treatment should improve the acquisition of accurate data, the outcome of IOL selection, and the overall long-term visual outcome of surgery. To me, the latter is remarkable. Up until now, we’ve used steroids as the go-to drug.
Dr. McGee: It’s essential for us ODs to have an ocular optimization strategy before patients get to the surgeon. We must encourage our peers to put themselves in the driver’s seat and not wait on the surgeon to optimize the ocular surface.
Patients with DED want a treatment that feels good in their eye. The tolerability profile of MIEBO is excellent on installation site pain. There was only one adverse event across both pivotal studies which occurred at a rate higher than 2% and that was blurred vision at an incidence of 2.1%.13,14
Dr. Hom: In the GOBI and MOJAVE studies, the burning and stinging rate on instillation was 0.5%.13,14 That’s extremely low.
Dr. Barnett: And the discontinuation rate due to adverse events was 0.2%, or one patient.13,14
PATIENT WORKUPS AND PRESCRIBING MIEBO
Dr. McGee: Each of us has prepared a case study for a certain patient profile that is appropriate for MIEBO (see the accompanying sidebars). Additionally, with the current landscape of medications and devices that we have, how do you decide where MIEBO fits in our treatment plan and protocols?
Dr. Hom: Lid-wiper epitheliopathy is a sign of dryness. According to the most recent literature,16,17 a decrease in friction alleviates dryness. Because this drop is thought to reduce friction, it may work well for dry eye patients lid-wiper epitheliopathy without resorting to a steroid.
Dr. Barnett: Additionally, it was well-tolerated over a year-long study.18 This is important because DED is a chronic condition.
Dr. Thimons: I love that MIEBO can sustain relative homeostasis, and continued use should eventually lead to improvements in the corneal surface. Patients might not care about the drug’s mechanism of action, but they want to know if it’s going to work and if their vision will improve. I think MIEBO has a great potential to impact a large base of patients who have failed with other drugs or do not have their needs met fully.
Dr. Barnett: We need to consider the patient and make sure they have subjective improvement. Of course, we’d love to see objective improvement as well. It’s important and impressive that MIEBO remains in the tears for up to 6 hours based on the results of a nonclinical study.
Dr. Cunningham: Ultimately, I think the duration is going to lead to flexibility. I think the second thing to understand is that the current therapies we have, including steroids and immunomodulators, are excellent, but they don’t treat the root of the DED. MIEBO, however, gives us a long-term option to augment other therapies and provide long-term stability while we continue to work on the root causes of the pathophysiological defect. I really see MIEBO being prescribed with other therapies to keep patients a lot happier and as a standalone therapy for individuals with early to moderate to significant disease.
MIEBO FOR TREATMENT-NAÏVE PATIENTS WITH MGD AND DED SYMPTOMS
MILTON HOM, OD
I usually prescribe an allergy drop or topical antihistamine along with the dry eye medication because I practice in an area with heavy pollen all year round. Some say that we never have a winter in Southern California.
Patients with MGD who have gland obstruction need a mechanical or lid therapy. Fortunately, we have many options to choose from. Those who have inflammation need an antiinflammatory, which is usually all dry eye patients. Pflugfelder once said that inflammation is the cause and/or consequence of dry eye. I like to use one of each treatment category type (mechanical and/or antiinflammatory) to optimize patients’ results. Using both treatment categories is highly synergistic because you are attacking from two different mechanisms of action.
MIEBO is an option that provides an alternative to physical therapies—by reducing the evaporative load and stabilizing the tear film, it improves both signs and symptoms of DED in patients with a deficient lipid layer.
Dr. Thimons: We use lissamine dye on every patient who has at least one symptom of DED. Interestingly, most patients who come in with symptomatic DED and are either on one drug or looking for some form of treatment have zero lissamine stain. But when you look at the posterior lid, they have significant posterior lid disease, which is mechanistic and more likely evaporative. MIEBO automatically jumps into that position for me. If patients have purely evaporative DED, prescribing a steroid is relatively meaningless. MIEBO instantly became a first-line adjunctive therapy for at least 50% of my patients.
Dr. McGee: When patients come in with symptoms, do you use some type of questionnaire?
Dr. Thimons: Yes, the technicians administer a questionnaire. If their score is high enough, they automatically undergo DED testing prior to the preoperative examination.
Dr. Barnett: The protocol we use varies slightly with each doctor, but all involve a questionnaire and staining with fluorescein. I’m also a huge fan of lissamine green dye and checking tear breakup time (TBUT). I like to evaluate the patient from across the room to look for things like ptosis, rosacea, ocular rosacea, and rheumatoid arthritis. At the slit lamp, I look at patients with their eyelids closed to evaluate for incomplete eyelid closure, floppy eyelids, and Demodex blepharitis. I evaluate the lids and lashes, cornea, conjunctiva, and TBUT. If imaging is available, I evaluate the meibomian glands with meibography.
It is great to have a complete workup prior to treating patients, but symptoms like blurry or fluctuating vision and a reduced blink rate are some of the best points for treating the patient. When looking at the meibomian glands, it is very important to push on the lower eyelid to evaluate for nonobvious meibomian gland dysfunction.
MIEBO FOR PATIENTS WITH CONTACT LENS INTOLERANCE
MELISSA BARNETT, OD
A 27-year-old woman of Asian descent presents complaining of discomfort with her soft contact lenses. She currently uses artificial tears but has not used eyelid cleaners, warm compresses, or other eyelid therapy. The patient has an otherwise unremarkable medical history. She uses the computer throughout the day and does not take many breaks. Her blink rate is poor.
Vision in both eyes was good (20/20), and the corneal curvature was normal in both eyes. On slit-lamp examination, however, significant meibomian gland dysfunction was found in both eyes (2+ OD and 3+ OS). She had ocular rosacea, blepharitis with collarettes, and signs of allergic conjunctivitis in both eyes. There was no corneal staining, epithelial defects, or corneal infiltrates, and the posterior segment was normal.
Instead of a prescription ointment, warm compresses, and an eyelid cleaner, MIEBO is a great first-line therapy for patients such as this one. Additionally, I like to counsel these patients about the benefits of taking breaks from the computer and the use of omega-3 fatty acids.
MIEBO gives us the opportunity to simplify DED therapy for these patients. The drug must be instilled 30 minutes prior to contact lens placement on the eye, but it may help reduce contact lens discomfort and contact lens dropout. It’s a unique option for these patients.
Dr. Hom: Studies have shown that frequency is an accurate predictor of the severity of DED.19 We ask patients how often they get dryness (sometimes, frequently, or always). Sometimes typically correlates with mild DED, frequently with moderate DED, and always with severe DED.
Then, we do a slit-lamp exam and lid expression to determine whether the patient has aqueous deficient or evaporative DED. More than likely, it includes an evaporative component. If I could do only one test, it would be lid expression.
Dr. Barnett: I think MIEBO is great for mild cases, and I prescribe it as an early intervention, even before artificial tears. Artificial tears with lipids or a higher viscosity might last longer than other tears, but they might blur vision and reduce contrast sensitivity.
Dr. Cunningham: Believe it or not, I don’t recommend or prescribe artificial tears because they don’t help the disease state. For me, MIEBO is a real step up in what we’re trying to do with the two pillars of DED.
We tell patients who come in for cataract surgery with a poor ocular surface that there are two steps to treating their DED. The first step is to deal with the inflammation. The second step is the lids. I think MIEBO is a groundbreaking product to address cataract patients who don’t get enough meibum spread over their eyes.
Dr. Barnett: I think it goes beyond cataract patients, too. Think about those who wear contact lenses; have mild to moderate dry eye disease or mild, moderate, or severe MGD; patients with a poor lipid layer; and those who use digital devices frequently. So many different patient types are well-suited for MIEBO.
MASTERING THE PATIENT CONVERSATION
Dr. McGee: Jim, how do you talk to patients about MIEBO with the limited amount of time we have to educate our patients?
Dr. Thimons: Most of my patients are excited to learn about a new treatment that can address their symptoms. It is a short conversation, much like my omega-3 conversation. I tell them that we need to improve the oil function to make their eye more comfortable. I say, “I want your windshield wiper to make a nice clean sweep over the surface of your eye, just like it would on a car. We’re going to provide you with that with MIEBO.” It’s as simple as that.
Dr. Barnett: It’s also important to talk about meibomian glands, the lipid layer, and tear film homeostasis. When it comes to contact lens wearers, it might be a little bit more complicated for them to use it 30 minutes prior to contact lens application, so I like to talk about that as well.
Dr. Cunningham: We have a growing patient base that doesn’t want to rely on medical therapies. I’ve found that patients appreciate that MIEBO works in a different way than other DED prescription therapies. They also respond positively to my explanation of its shield effect. I say, “Nothing else we’ve ever had has been able to provide a shield effect to your eyes. It’s a very well-tolerated initial therapy."
MIEBO is becoming my go-to when patients’ current therapy isn’t giving enough relief. In fact, it can change the cycle of DED and simplify treatment.
Dr. McGee: MIEBO has a unique mechanism of action that targets the evaporative component of DED. It works on an entirely different level by directly targeting the evaporative component of DED, and clinicians should keep an open mind when deciding what patients could benefit from the treatment. The potential of this new treatment is exciting.
1. Badian RA, Utheim TP, Chen X, et al. Meibomian gland dysfunction is highly prevalent among first-time visitors at a Nrwegian dry eye specialist clinic. Sci Rep. 2021;11:23412.
2. Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31:472-478.
3. Messmer EM. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch Arztebl Int. 2015;112(5):71-81.
4. Craig JP, Nelson JD, Azar DT, et al. TFOS DEWS II report executive summary. Ocul Surf. 2017;15:802-812.
5. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15:276-283.
6. Rabensteiner DF, Aminfar H, Boldin I, Schwantzer G, Horwath-Winter J. The prevalence of meibomian gland dysfunction, tear film and ocular surface parameters in an Austrian dry eye clinic population. Acta Ophthalmol. 2018;96:e707-e711.
7. Lemp MA, Bron AJ, Baudouin C, et al. Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol. 2011;151(5):792-798.e1.
8. Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II Epidemiology Report. Ocul Surf. 2017;334-368.
9. Paulsen AJ, Cruickshanks KJ, Fischer ME, et al. Dry eye in the beaver dam offspring study: prevalence, risk factors, and health-related quality of life. Am J Ophthalmol. 2014;157:799-806.
10. 2020 dry eye products market report: a global analysis for 2019 to 2025. Market Scope. April 2021. Accessed August 28, 2023. market-scope.com/pages/reports/250/2020-ophthalmic-landscape-report-global-analysis-for-2019-to-2025-april-2021#reports.
11. IQVIA data, July 2021.
12. Wolffsohn JS, Lingham G, Downie LE, et al. TFOS Lifestyle: impact of the digital environment on the ocular surface. The Ocular Surface. 2023;28:213-252.
13. Tauber J, Berdy GJ, Wirta DL, Krösser S, Vittitow JL; GOBI Study Group. NOV03 for dry eye disease associated with meibomian gland dysfunction: results of the randomized phase 3 GOBI study. Ophthalmology. 2023;130(5):516-524.
14. Sheppard JD, Kurata F, Epitropoulos AT, Krösser S, Vittitow JL; MOJAVE Study Group. NOV03 for signs and symptoms of dry eye disease associated with meibomian gland dysfunction: the randomized phase 3 MOJAVE study. Am J Ophthalmol. 2023;252:265-274.
15. Hovanesian J, Epitropoulos A, Donnenfeld ED, Holladay JT. The effect of lifitegrast on refractive accuracy and symptoms in dry eye patients undergoing cataract surgery. Clin Ophthalmol. 2020;14:2709-2716.
16. Nichols JJ, Lievens CW, Bloomenstein MR, et al. Dual-polymer drops, contact lens comfort, and lid wiper epitheliopathy. Opt Vis Sci. 2016;93(8):979-986.
17. Lin MC, Yeh TN. Mechanical complications induced by silicone hydrogel contact lenses. Eye Contact Lens. 2013;39(1):115-124.
18. Protzko EE, Segal BA, Korenfeld MS, Krosser S, Vittitow JL. Long-term safety and efficacy of perfluorohexyloctane ophthalmic solution for the treatment of patients with dry eye disease: the KALAHARI study. Cornea. 2023.
19. Hom M, De Land P. Self-reported dry eyes and diabetic history. Optometry. 2006;77(11):554-558.
MBO.0579.USA.23
INDICATION AND IMPORTANT SAFETY INFORMATION
Indication
MIEBO® (perfluorohexyloctane ophthalmic solution) is indicated for treatment of the signs and symptoms of dry eye disease.
IMPORTANT SAFETY INFORMATION
MIEBO should not be administered while wearing contact lenses. Contact lenses should be removed before use and for at least 30 minutes after administration of MIEBO.
- Instruct patients to instill one drop of MIEBO into each eye four times daily
- The safety and efficacy in pediatric patients below the age of 18 have not been established
- The most common ocular adverse reaction was blurred vision (1% to 3% of patients reported blurred vision and conjunctival redness)
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please see full Prescribing Information for MIEBO here.