Oculis Announces Positive Phase 2 Results for OCS-05 in Acute Optic Neuritis as a Potential Neuroprotective Therapy
Oculis announced positive topline results for OCS-05 in the phase 2 ACUITY trial for patients with acute optic neuritis. The trial met its primary endpoint of safety and achieved statistical significance on several key efficacy-based secondary endpoints.
The phase 2 ACUITY (Acute OptiC NeUrITis of DemYelinating Origin) trial was a randomized, double-blind, placebo-controlled, multicenter trial, designed to evaluate OCS-05 (2mg/kg/day or 3mg/kg/day) administered intravenously once-daily for 5 days in patients with acute optic neuritis also receiving steroid. The study randomized 36 patients with recent onset (visual loss symptoms) of unilateral acute optic neuritis with a demyelinating origin, of which 33 patients received treatment and were included in the prespecified modified intent-to-treat (mITT) analysis.
Positive results from the ACUITY trial showed that OCS-05 achieved primary safety endpoint in addition to highlighting neuroprotective structural benefit and the ability to improve visual function in patients suffering from acute optic neuritis, according to Oculis.
Primary Endpoint was Safety-Based: The percentage of patients with a shift from normal (baseline) to abnormal in electrocardiogram (ECG) parameters after study drug administration until Visit 4 (Day 15) was measured to evaluate cardiac safety. The results showed no difference in the percentage of patients with abnormal ECG parameters between the two treatment arms.
Two patients in the OCS-05 arms (2 and 3 mg/kg/day) and one patient in the placebo arm had a shift from normal to abnormal in any ECG measures between baseline and Visit 4 (Day 15), both equivalent to 12.5%. Events observed in the OCS-05 arms were mild and transient and qualified as not clinically significant by central review reading center.
Secondary Efficacy Endpoints Assessed Changes in Retinal Structure: Optical Coherence Tomography (OCT) imaging was used to objectively measure the thickness of two different retinal segments in the affected eye to evaluate the potential neuroprotective effects of OCS-05 compared to placebo: 1) Ganglion Cell-Inner Plexiform Layer (GCIPL) and 2) Retinal Nerve Fiber Layer (RNFL). Results showed:
A 43% improvement in GCIPL thickness mean change from baseline in favor of OCS-05 (3mg/kg/day) + steroid compared to placebo + steroid at month 3 which was maintained through month 6 with P-values* of 0.049 and 0.052 at 3 and 6 months, respectively.
A 28% improvement in RNFL thickness mean change from baseline in favor of OCS-05 (3mg/kg/day) + steroid compared to placebo + steroid at month 3 reaching 30% improvement at month 6 with P-values* of 0.045 and 0.033 at 3 and 6 months, respectively.
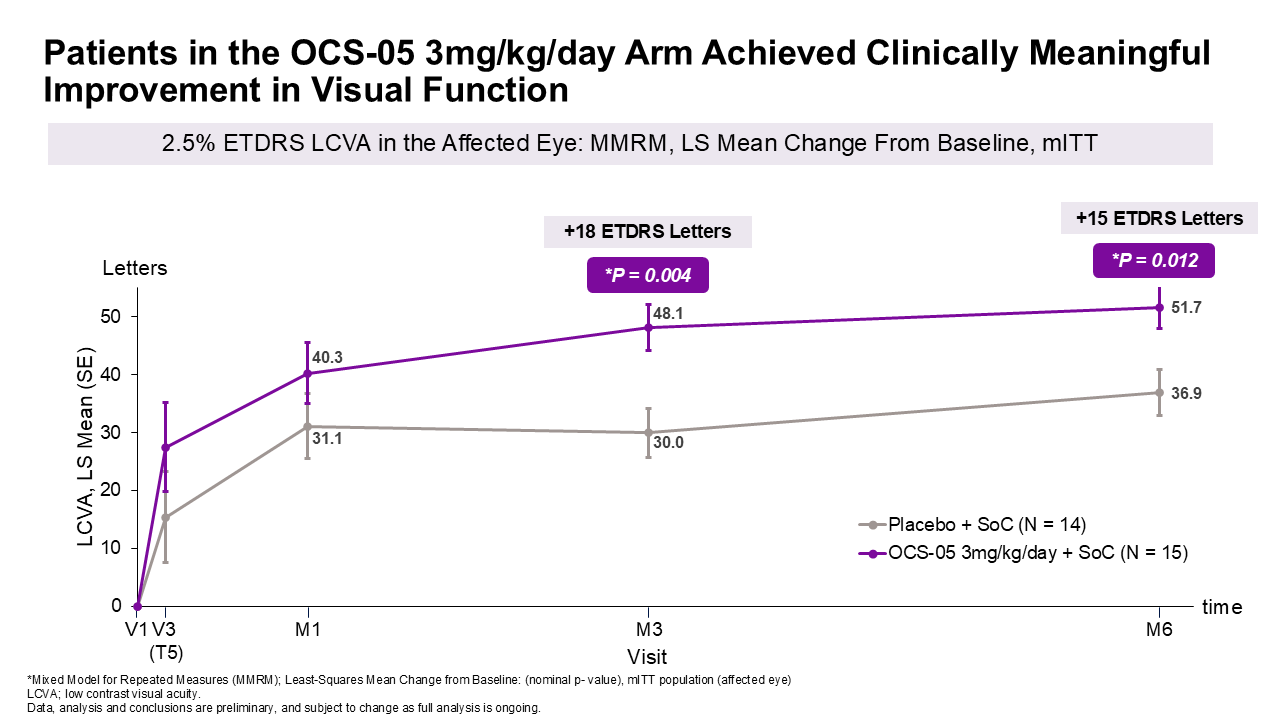
Secondary Efficacy Endpoint Assessed Changes in Visual Function:
Changes in 2.5% ETDRS low contrast letter acuity (LCVA) were measured to assess visual function improvement. Results showed:
A favorable difference in LCVA mean change from baseline of approximately 18 letters at month 3 and approximately 15 letters at month 6 with OCS-05 (3 mg/kg/day) + steroid compared to placebo + steroid, with P-values^ of 0.004 and 0.012 at 3 and 6 months, respectively.
There were no drug-related serious adverse events (SAEs); and no AEs leading to drug withdrawal or study discontinuation. The most frequently reported drug related AEs > 10% in the OCS-05 (2 or 3 mg/kg/day) + steroid treatment group were headache: 2 patients (10.5%), and acne: 2 patients (10.5%).
“These positive safety and efficacy results from ACUITY represent a significant milestone in bringing the first potential neuroprotective treatment in ophthalmology to patients," Riad Sherif, MD, Chief Executive Officer of Oculis, said in a company news release. "The improvement in vision is especially encouraging, and the consistent improvement in retinal structure highlights the therapeutic potential of OCS-05 across multiple ophthalmic and neurological conditions. We are excited to further advance OCS-05’s development in acute optic neuritis, while actively exploring its potential in additional neuro-ophthalmic indications with the aim to deliver a first-in-class neuroprotective treatment option to patients.”
"Steroids have been used to treat the inflammation seen in acute optic neuritis, but don’t prevent persistent visual impairments or reduce structural loss. There remains a critical unmet need for neuroprotective therapies to preserve vision and the potential neuroprotective properties of OCS-05 observed in the ACUITY trial and its impact on visual function could offer significant hope for patients," said Mark Kupersmith, MD, Professor, Vice chair translational research, Chair NORDIC at Icahn School of Medicine at Mount Sinai Hospital, New York. "These results, if replicated in larger clinical trials, could have profound implications, not only for this condition, but potentially for MS and other optic nerve disorders as well as glaucoma.”
OCS-05 has received orphan drug designation from the FDA and the European Medicines Agency (EMA) for acute optic neuritis. There are currently no approved therapies specifically indicated for acute optic neuritis and despite steroids being used to treat inflammation and improve recovery, steroids are unable to provide neuroprotection to prevent vision loss. The investigational new drug (IND) application for OCS-05 has also been cleared by the FDA, enabling the initiation of clinical development in the United States to support the global potential of OCS-05.
