Demodex blepharitis (DB)—inflammation caused by overpopulation of Demodex mites in the eyelash follicles—is particularly common and dramatically underdiagnosed.1 It's estimated that approximately 25 million eye care patients in the United States are affected by DB.2 This includes patients with cataracts, meibomian gland dysfunction (MGD), dry eye disease (DED), glaucoma, and other comorbidities, as well as soft contact lens wearers. In fact, 93% of patients (26 out of 28 patients) with intolerance to soft contact lens wear were found to have evidence of Demodex.3 After demonstrating safety and tolerability, XDEMVY® (lotilaner ophthalmic solution 0.25%; Tarsus), the first and only available FDA-approved treatment for DB, received its approval in August 2023. Here, I will discuss how to deliver an informed diagnosis of DB to patients and detail the efficacy and safety data from the SATURN studies that led to the approval of XDEMVY®.
DB Diagnostic Criteria and Patient Communication
There are multiple diagnostic criteria for DB, but I look for three specific signs. First, inflammation produced by Demodex mites generates a chemical reaction that can cause hyperemia and telangiectasias of the vessels, which may lead to discomfort for patients. Second, these mites can cause damage to the shaft of the eyelashes. And, many patients may begin to experience loss of their eyelashes and other related issues. Overall, Demodex mites can cause inflammation of the eyelids, producing red, itchy, irritated eyelids, surface irritation, eyelash misdirection, eyelash loss, and fluctuating vision.
My diagnosis procedure includes examination for mild to severe DB. I have patients sit at the slit lamp, look down, and I check the lash line for collarettes. Collarettes are a pathognomonic sign for DB, and that’s when I know to treat. I also make sure to evaluate all my MGD patients for DB.
When relaying my diagnosis to patients, I don’t skirt around it; I tell them they have mites. However, it’s important to reassure patients this is not a transmissible, contagious disease, and that millions of people have experienced this condition. Leading with this narrative is not only helpful to encourage patients to begin treatment, but it will also help reduce chair time to ensure the conversation is productive and focused on corrective treatment.
SATURN Trials: XDEMVY® Efficacy Outcomes
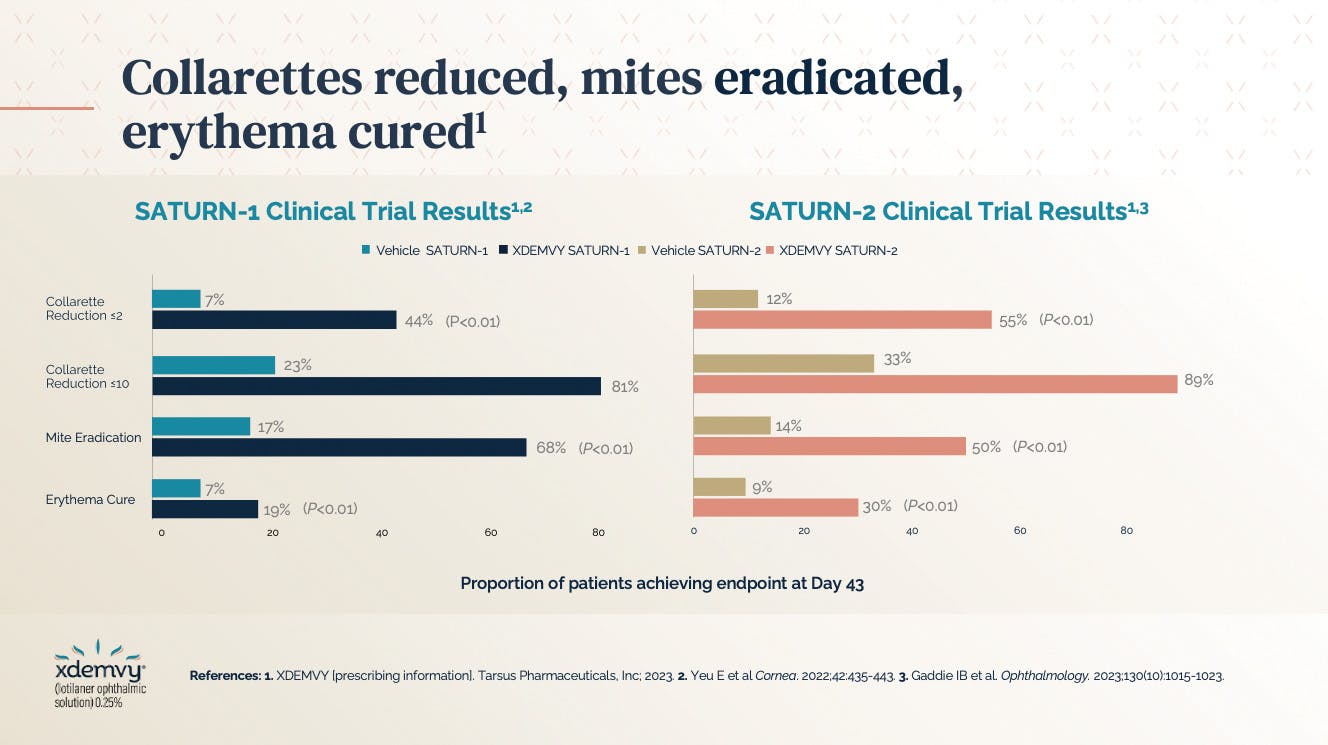
As of late 2023, we now have an FDA-approved treatment for DB. Lotilaner, the active ingredient in XDEMVY®, is a lipophilic agent in an aqueous drop that acts specifically via mite gamma-aminobutyric acid-gated (GABA-gated) chloride channels to target, paralyze, and kill Demodex mites.4-6 This treatment demonstrated its clinical efficacy and safety within 6 weeks in two clinical trials—SATURN-1 (phase 2b/3) and SATURN-2 (phase 3)—to receive FDA approval. Both trials examined the safety and efficacy of lotilaner ophthalmic solution 0.25% compared with vehicle for the treatment of Demodex blepharitis. The results between the two trials were similar, with reduced evidence of collarettes and the eradication of mites and erythema (Figure 1).
Fifty percent of patients in the studies achieved a collarette Grade 0 at day 43. Both studies demonstrated that 81% to 89% of patients had collarette reduction to less than 10, meaning four out of five patients achieved grade 1 or better in the 6 weeks of use timeframe, which is remarkable.6,7 Additionally, erythema was cured in about 25% of patients between the SATURN-1 and SATURN-2 trials. The recommended dosage is one drop per eye, twice a day, approximately 12 hours apart, over 6 weeks.4 The most common adverse reaction with XDEMVY® was instillation site stinging and burning which was reported in 10% of patients.4 Other ocular adverse reactions reported in less than 2% of patients included chalazion/hordeolum and punctate keratitis.4
In addition to the efficacy of lotilaner in its clinical trials, patients reported that it was comfortable to use. In fact, a tagline I often use for this treatment is: “XDEMVY® is tough on mites and easy on patients.” In the SATURN-1 and SATURN-2 trials, drop comfort was assessed at all visits up to day 43, and most patients in the trial found the drop to be neutral to very comfortable (averaging the responses for the 6-week period) (Figure 2).6,7 In conclusion, lotilaner ophthalmic solution 0.25% proved to be a safe and effective treatment for patients with Demodex blepharitis.
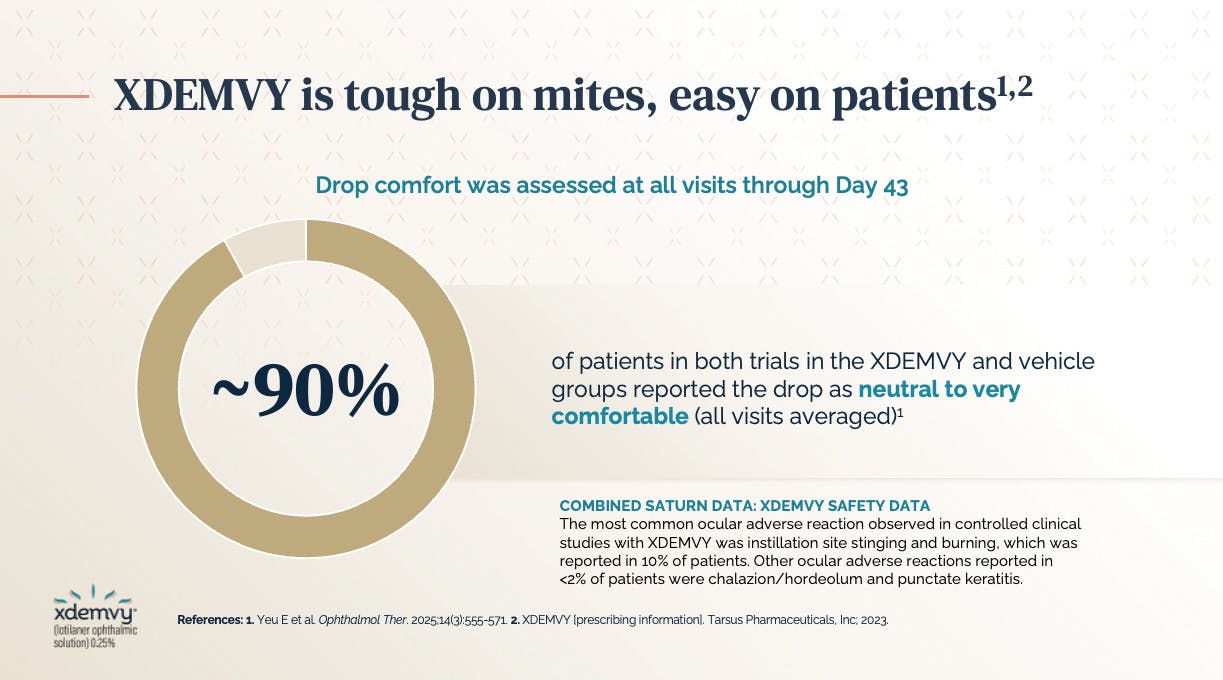
Figure 2. In the SATURN-1 and SATURN-2 trials, most patients found XDEMVY® to be neutral to very comfortable.
ERSA & RHEA: db and Meibomian Gland disease Trial Data
In addition to the SATURN trials, two separate pilot studies were conducted— ERSA and RHEA—in DB patients with MGD as well. These two studies were separate, randomized pilot trials with 21 patients enrolled in ERSA and 12 patients enrolled in RHEA, designed to explore the safety and efficacy of XDEMVY® in this specific patient population.8 Both trials had similar eligibility criteria and study endpoints of safety/tolerability, patient reported outcomes, collarette grade reduction, and meibomian gland function/expressibility. Participants had to have MGD and mite infestation in the same eye. They had to have at least 11 eyelashes with collarettes, otherwise known as grade 2, 3, or 4. Patients also needed to have at least one mite present based on epilated lashes from the upper and lower eyelids.
An initial question raised in these studies was how to measure MGD, and the determination was with meibomian glands yielding any liquid secretions (MGYLS). Overall, 15 meibomian glands were expressed in the lower eyelid and the secretions graded. For example, MGYLS means the patient had cloudy or clear liquid expressed from a gland that reflected a score of 2 and 3 respectively. A score of 0 meant no expression, and a score of 1 was solid expression. A meibomian gland secretion score of between 12 and 32 indicated MGD. Additionally, patients had to have at least grade-1 erythema, a tear breakup time of less than 10 seconds, and at least one-third of their meibomian glands had to be intact in this assessment.
At baseline in the ERSA study, 21 eyes of 21 patients had a mean of 7 expressible glands yielding any liquid secretion. At the end of 6 weeks, 10.8 out of 15 glands per 20 eyes of 20 patients (1 dropped out) yielded any liquid secretions, which is a 54% improvement in MGYLS.8 Furthermore, the ERSA trial results demonstrated that about 55% of patients had a reduction in collarettes to grade zero at the end of 6 weeks. Patients reported an improvement in fluctuating vision, itching, and redness when compared to baseline (Figure 3). At Day 43 in the RHEA study, the vehicle group did not show statistically significant improvement in mean collarette grade or in number of glands yielding any liquid secretion when compared to baseline. During this same period, the vehicle cohort also did not demonstrate statistically significant improvements in patient-reported fluctuating vision, itching, or redness when compared with baseline (P > 0.1).8
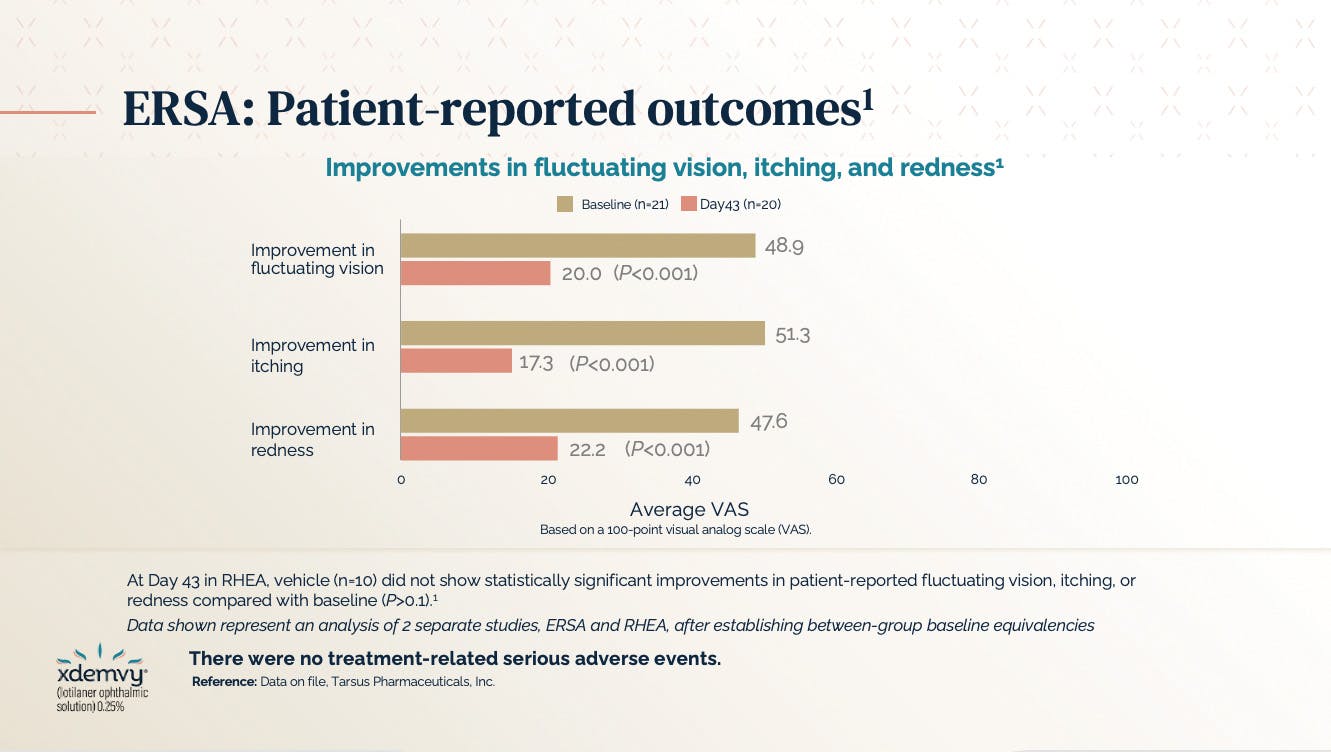
Figure 3. Patient-reported outcomes from the ERSA trial showed improvement in fluctuating vision, itching, and redness.
Conclusion
In my opinion, XDEMVY® is a great option for our DB patients, and as the data demonstrate, it works. The drops are tolerable and have no contraindications. If patients are contact lens wearers, I recommend that they wait 15 minutes after instilling the drop before putting the lenses back in the eyes.
For me, XDEMVY® works: I see collarettes and I prescribe it. In the beginning, I would have patients return for re-evaluation in 2-3 weeks to ensure they are on the right track and are tolerating it well. At this point, my confidence in the treatment is strong, and I prescribe it and ask the patient to contact us if they are experiencing any issues. Over the 6-week treatment course, I have been impressed in the efficacy of the medication, it matches that which we learned in the clinical trials. I encourage full treatment to ensure we are doing our best to eradicate the mites. Outside of drop instillation-related transient stinging and burning, patients are quite thrilled with the results. In the event of a recurrence in infestation, I am confident in prescribing XDEMVY.
1. Trattler W, Karpecki P, Rapoport Y, et al. The prevalence of Demodex blepharitis in US eye care clinic patients as determined by collarettes: a pathognomonic sign. Clin Ophthalmol. 2022;16:1153-1164.
2. O'Dell L, Dierker DS, Devries DK, et al. Psychosocial impact of demodex blepharitis. Clin Ophthalmol. 2022; 16:2979-2987.
3. Tarkowski W, Moneta-Wielgoś J, Młocicki D. Demodex sp. as a potential cause of the abandonment of soft contact lenses by their existing users. Biomed Res Int. 2015;2015:259109.
4. XDEMVY [prescribing information]. Tarsus Pharmaceuticals, Inc; 2023.
5. Toutain CE, Seewald W, Jung M. The intravenous and oral pharmaco-kinetics of lotilaner in dogs. Parasit Vectors. 2017;10:522.
6. Yeu E, Wirta DL, Karpecki P, et al. Lotilaner ophthalmic solution, 0.25%, for the treatment of Demodex blepharitis: results of a prospective, randomized, vehicle-controlled, double-masked, pivotal trial (Saturn-1). Cornea. 2022;42:435-443.
7. Gaddie IB, Donnenfeld ED, Karpecki P, et al. Lotilaner ophthalmic solution 0.25% for Demodex blepharitis: randomized, vehicle-controlled, multicenter, phase 3 trial (Saturn-2). Ophthalmology. 2023. doi:10.1016/j.ophtha.2023.05.030.
8. McGee S, Vollmer P, Shen Lee B, et al. Lotilaner Ophthalmic Solution, 0.25% for the Treatment of Demodex Blepharitis Patients with Meibomian Gland Disease. Oral presentation at: Southern Council of Optometrists 2025; February 26-March 2, >2025; Atlanta, GA. 2. Lane SS, et al. Cornea. 2012;31(4):396-404.
INDICATIONS AND USAGE
XDEMVY is indicated for the treatment of Demodex blepharitis.
Important Safety Information:
WARNINGS AND PRECAUTIONS
Risk of Contamination: Do not allow the tip of the dispensing container to contact the eye, surrounding structures, fingers, or any other surface in order to minimize contamination of the solution. Serious damage to the eye and subsequent loss of vision may result from using contaminated solutions.
Use with Contact Lenses: XDEMVY contains potassium sorbate, which may discolor soft contact lenses. Contact lenses should be removed prior to instillation of XDEMVY and may be reinserted 15 minutes following its administration.
ADVERSE REACTIONS: The most common adverse reaction with XDEMVY was instillation site stinging and burning which was reported in 10% of patients. Other ocular adverse reactions reported in less than 2% of patients were chalazion/hordeolum and punctate keratitis.
Please view prescribing infomation at xdemvy.com.
© 2025 Tarsus Pharmaceuticals 09/25 US--2500437